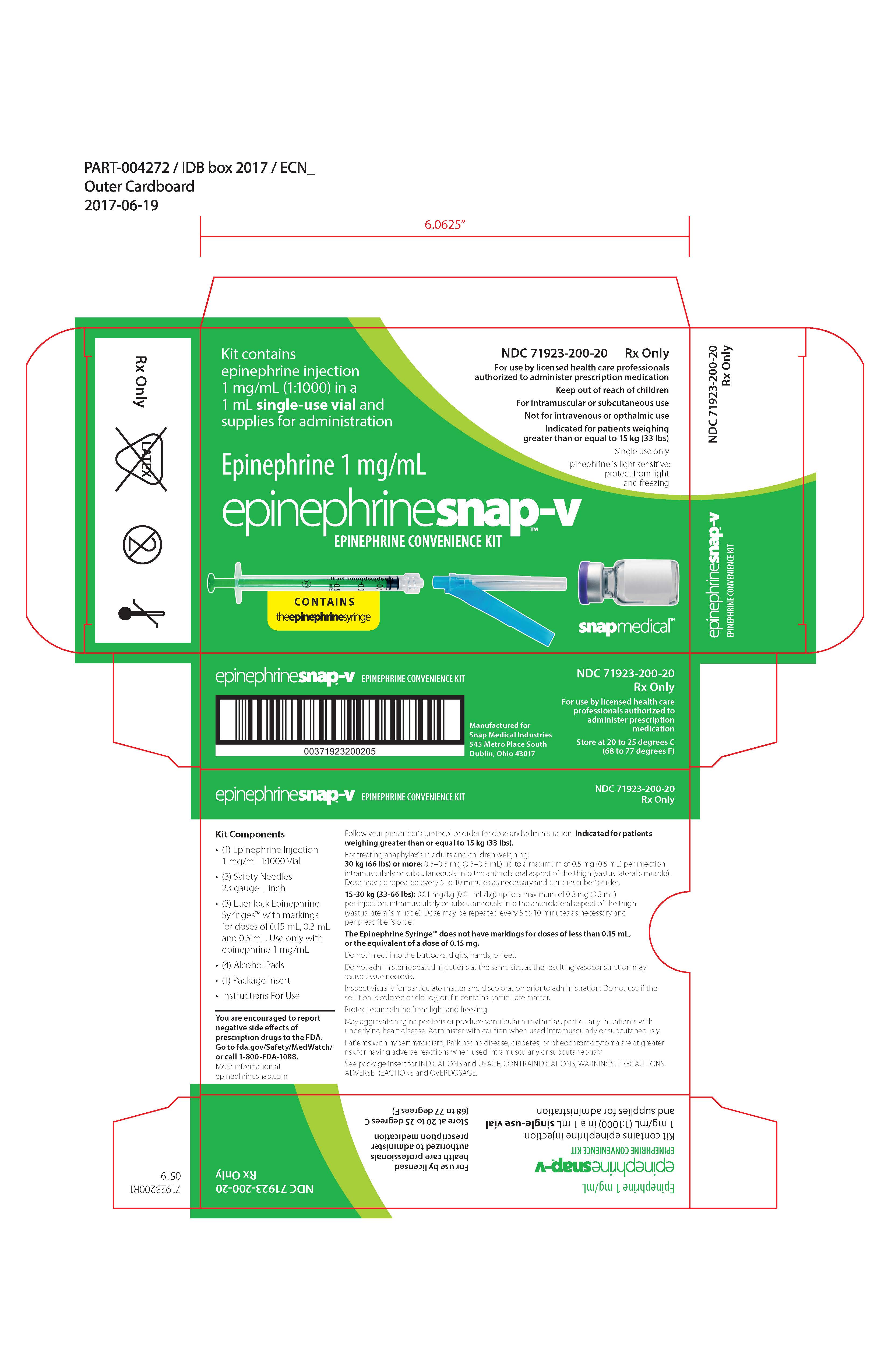
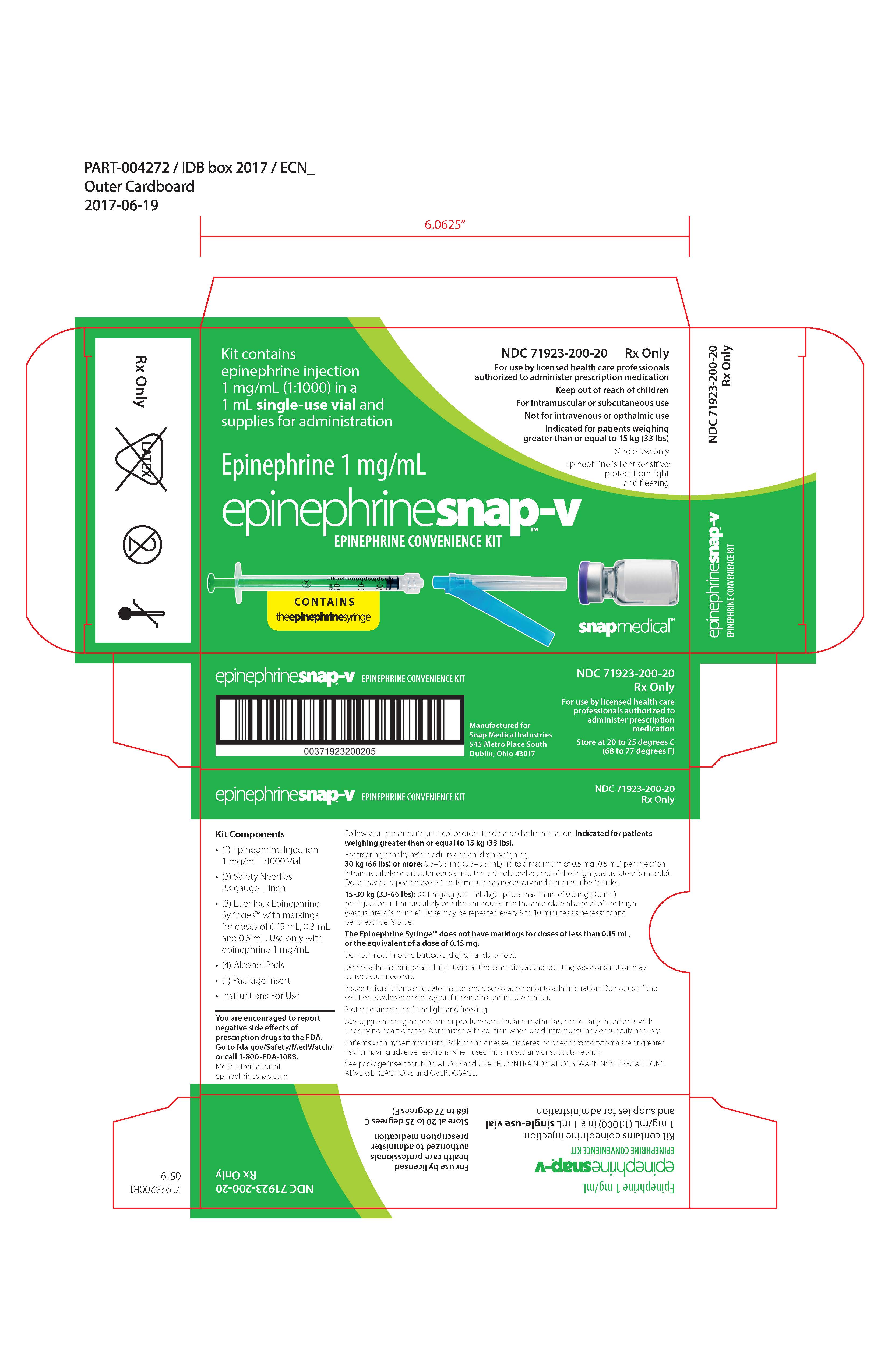
Label: EPINEPHRINESNAP-V- epinephrine convenience kit kit
-
Contains inactivated NDC Code(s)
NDC Code(s): 42023-159-25, 68599-5804-1, 71923-200-20 - Packager: Snap Medical Industries
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated January 13, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use ADRENALIN safely and effectively. See full prescribing information for ADRENALIN. ADRENALIN ...
-
Table of ContentsTable of Contents
-
1 ADRENALIN
® INDICATIONS AND USAGE
Adrenalin - ® is available as a single-use 1 mL vial and a multiple-use 30 mL vial for intramuscular and subcutaneous use. Emergency treatment of ...
-
2 DOSAGE AND ADMINISTRATIONInject Adrenalin - ® intramuscularly or subcutaneously into the anterolateral aspect of the thigh, through clothing if necessary. When administering to a child, to minimize the risk of injection ...
-
3 DOSAGE FORMS AND STRENGTHSAdrenalin - ® 1 mg/mL epinephrine injection, 1 mL solution in a single-use clear glass vial and 30 mL solution in a multiple-dose amber glass vial ...
-
4 CONTRAINDICATIONSNone.
-
5 WARNINGS AND PRECAUTIONS5.1 Incorrect Locations of Injection - Injection into the anterolateral aspect of the thigh (vastus lateralis muscle) is the most appropriate location for administration because of its location ...
-
6 ADVERSE REACTIONSCommon adverse reactions to systemically administered epinephrine include anxiety, apprehensiveness, restlessness, tremor, weakness, dizziness, sweating, palpitations, pallor, nausea and vomiting ...
-
7 DRUG INTERACTIONSEpinephrine should be administered cautiously to patients taking other sympathomimetic agents because of the possibility of additive effects. Patients who are concomitantly receiving cardiac ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Teratogenic Effects: Pregnancy Category C. There are no adequate and well-controlled studies in pregnant women. Epinephrine should be used during pregnancy only if the ...
-
10 OVERDOSAGEOverdosage of epinephrine may produce extremely elevated arterial pressure, which may result in cerebrovascular hemorrhage, particularly in elderly patients. Overdosage may also result in ...
-
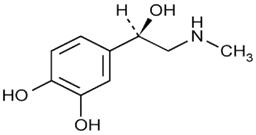
11 DESCRIPTIONAdrenalin - ® (epinephrine injection, USP) is a clear, colorless, sterile solution containing 1 mg/mL epinephrine, packaged as 1 mL of solution in a single-use ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Epinephrine acts on both alpha and beta-adrenergic receptors. 12.2 Pharmacodynamics - Through its action on alpha-adrenergic receptors, epinephrine lessens the ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Long- term studies to evaluate the carcinogenic potential of epinephrine have not been conducted. Epinephrine and other catecholamines ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGAdrenalin - ® 1 mL Single-Use Vials: Each carton contains 25 single-use vials containing 1 mL Adrenalin ...
-
17 PATIENT COUNSELING INFORMATIONAdvise patients or their caregivers about common adverse reactions associated with the use of epinephrine including an increase in heart rate, the sensation of a more forceful heartbeat ...
-
SPL UNCLASSIFIED SECTIONDistributed by: Par Pharmaceutical - Chestnut Ridge, NY 10977 - Adrenalin - ® is a registered trademark of Par Sterile ...
-
INSTRUCTIONS FOR USEALCOHOL PREP PAD- isopropyl alcohol swab - Drug Facts - Active ingredient - Isopropyl Alcohol 70% v/v - Purpose - First Aid Antiseptic - Use - For preparation of the skin prior to an ...
-
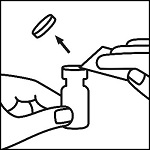
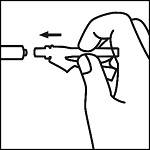
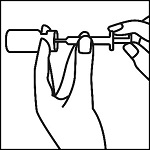
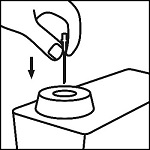
Instructions for Use
EpinephrineSnap™-V - Epinephrine convenience kit - Rx Only - NDC 71923-200-20 - Single use only - For intramuscular or subcutaneous use - Not for intravenous or ophthalmic use - Indicated for patients ...
-
PRINCIPAL DISPLAY PANELPRINCIPAL DISPLAY PANEL - 1 mL Vial Label - NDC 42023-159-25 - Rx Only - Adrenalin - ® (epinephrine injection, USP) 1 mg/mL - NOT for Ophthalmic ...
-
PRINCIPAL DISPLAY PANELPRINCIPAL DISPLAY PANEL – PACKET LABEL - NDC 68599-5804-1 - MCKESSON - Alcohol Prep Pad - STERILE | SATURATED WITH - 70% v/v ISOPROPYL ALCOHOL - 1.2" x 2.6" (3 ...
-
PRINCIPAL DISPLAY PANELPRINCIPAL DISPLAY PANEL – Carton Label - NDC 71923-100-20 - Rx Only - For use by licensed health care professionals authorized to administer prescription medication - Keep out of reach of children - For ...
-
INGREDIENTS AND APPEARANCEProduct Information