Label: EXEMESTANE tablet, film coated
- NDC Code(s): 71921-190-09, 71921-190-33
- Packager: Florida Pharmaceutical Products, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated June 19, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use EXEMESTANE TABLETS safely and effectively. See full prescribing information for EXEMESTANE TABLETS. EXEMESTANE tablets, for oral ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE1.1 Adjuvant Treatment of Postmenopausal Women - Exemestane is indicated for adjuvant treatment of postmenopausal women with estrogen-receptor positive early breast cancer who have received two ...
-
2 DOSAGE AND ADMINISTRATION2.1 Recommended Dose - The recommended dose of exemestane in early and advanced breast cancer is one 25 mg tablet once daily after a meal. adjuvant treatment of postmenopausal women with ...
-
3 DOSAGE FORMS AND STRENGTHSExemestane Tablets, USP are white or almost white round film-coated tablets, debossed with 111 on one side. Each tablet contains 25 mg of exemestane, USP.
-
4 CONTRAINDICATIONSExemestane is contraindicated in patients with a known hypersensitivity to the drug or to any of the excipients.
-
5 WARNINGS AND PRECAUTIONS5.1 Reductions in Bone Mineral Density (BMD) Reductions in bone mineral density (BMD) over time are seen with exemestane use. Table 1 describes changes in BMD from baseline to 24 months in ...
-
6 ADVERSE REACTIONSThe following clinically significant adverse reactions are described elsewhere in the labeling: Reductions in Bone Mineral Density (BMD) [see - Warnings and Precautions (5.1) ...
-
7 DRUG INTERACTIONSDrugs That Induce CYP 3A4 - Co-medications that induce CYP 3A4 (e.g., rifampicin, phenytoin, carbamazepine, phenobarbital, or St. John's wort) may significantly decrease exposure to exemestane ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Based on findings in animal studies and its mechanism of action - ,exemestane can cause fetal harm when administered to a pregnant woman - [see ...
-
10 OVERDOSAGEClinical trials have been conducted with exemestane given as a single dose to healthy female volunteers at doses as high as 800 mg and daily for 12 weeks to postmenopausal women with advanced ...
-
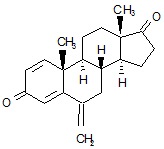
11 DESCRIPTIONExemestane Tablets, USP for oral administration contain 25 mg of exemestane, USP, an irreversible, steroidal aromatase inactivator. Exemestane, USP is chemically described as ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Breast cancer cell growth may be estrogen-dependent. Aromatase is the principal enzyme that converts androgens to estrogens both in pre- and postmenopausal women. While ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - A 2-year carcinogenicity study in mice at doses of 50, 150, and 450 mg/kg/day exemestane (gavage), resulted in an increased incidence ...
-
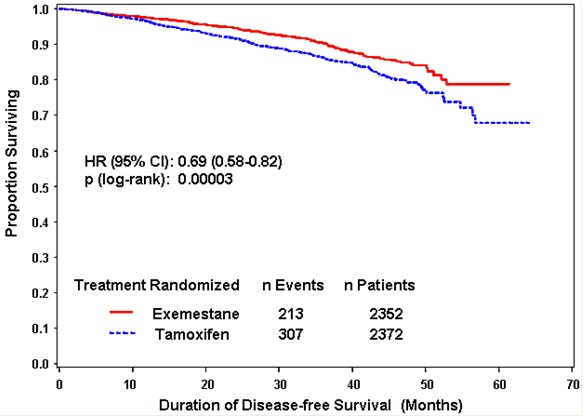
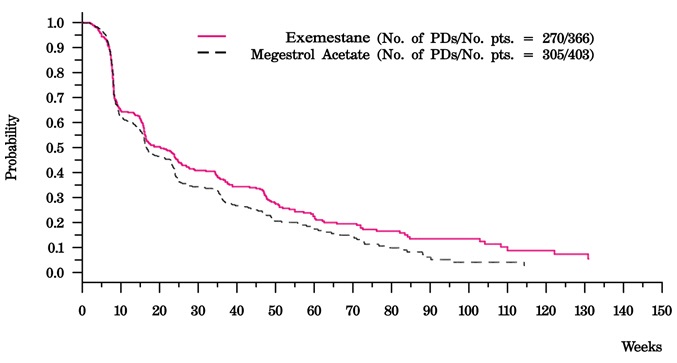
14 CLINICAL STUDIES14.1 Adjuvant Treatment in Early Breast Cancer - The Intergroup Exemestane Study 031 (IES) was a randomized, double-blind, multicenter, multinational study comparing exemestane (25 mg/day) vs ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGExemestane Tablets, USP are white or almost white round film-coated tablets, debossed with 111 on one side. Each tablet contains 25 mg of exemestane, USP. Exemestane Tablets, USP are packaged in ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA - -approved patient labeling (Patient Information). Bone Effects - Advise patients that exemestane lowers the level of estrogen in the body. This may ...
-
SPL UNCLASSIFIED SECTIONManufactured by: Qilu Pharmaceutical Co., Ltd. Jinan, 250104, China - Manufactured for: Florida Pharmaceutical Products, LLC - Boca Raton, 33487, FL, USA - Revised: 1/2022 - Code number ...
-
PATIENT PACKAGE INSERTPatient Information - Exemestane (EX-e-MES-tane) Tablets, USP - This Patient Information has been approved by the U.S. Food and Drug AdministrationRevised: 1/2022 - What is ...
-
PRINCIPAL DISPLAY PANEL - 25 mg Tablet Bottle LabelNDC 71921- 190-33 - Exemestane - Tablets, USP - 25 mg - FPP logo Rx only 30 tablets
-
INGREDIENTS AND APPEARANCEProduct Information