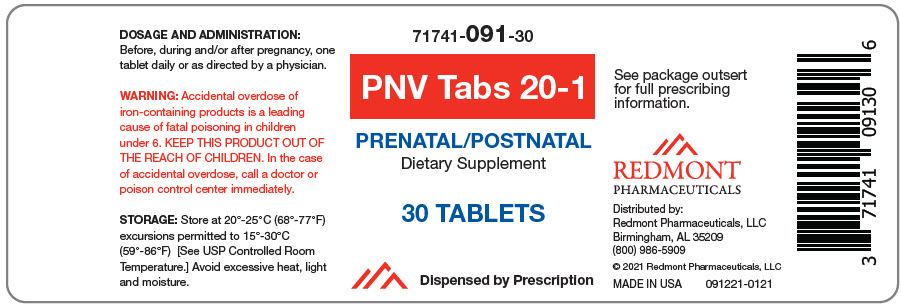
Label: PNV TABS 20-1- beta carotene, ascorbic acid, cholecalciferol, .alpha.-tocopherol acetate, pyridoxine hydrochloride, biotin, folic acid, levomefolate calcium, cyanocobalamin, calcium carbonate, magnesium oxide, ferrous bisglycinate, and potassium iodide tablet
- NHRIC Code(s): 71741-091-30
- Packager: Redmont Pharmaceuticals, LLC
- Category: DIETARY SUPPLEMENT
- DEA Schedule: None
- Marketing Status: Dietary Supplement
Drug Label Information
Updated January 21, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- HEALTH CLAIM
-
DESCRIPTION
PNV Tabs 20-1 is a prescription prenatal/postnatal multivitamin/multimineral dietary supplement.
Supplement Facts Serving size 1 Tablet Amount per Serving: %Daily Value - *
- Daily Value not established
Vitamin A (as Beta-Carotene) 300 mcg RAE 33% Vitamin C (as Ascorbic Acid) 60 mg 67% Vitamin D (as Cholecalciferol) 10 mcg 50% Vitamin E (as dl-Alpha Tocopherol Acetate) 4.5 mg (10 IU) 33% Vitamin B6 (as Pyridoxine HCl) 26 mg 1529% Biotin 0.280 mg 933% Folate 1.67 mg DFE 418% (from Folic Acid) 0.67 mg DFE * (from 5-Methyl Tetrahydrofolate, Calcium Salt) 1 mg DFE * Vitamin B12 (as Cyanocobalamin) 0.013 mg 542% Calcium (as Calcium Carbonate) 80 mg 6% Magnesium (as Magnesium Oxide) 25 mg 6% Ferrochel™ Iron (as Ferrous BisGlycinate Chelate) 20 mg 111% Iodine (as Potassium Iodide) 0.150 mg 100% OTHER INGREDIENTS: Microcrystalline Cellulose, Maltodextrin, Croscarmellose Sodium, Silicon Dioxide, Stearic Acid, Magnesium Stearate, Film Coating (Hydroxypropyl Methylcellulose, Polyethylene Glycol, Titanium Dioxide, FD&C Blue # 1)
Allergen: NONE
-
INDICATIONS
PNV Tabs 20-1 is a multivitamin/multimineral dietary supplement indicated for the use in improving the nutritional status of women throughout pregnancy and in the postnatal period for both lactating or non-lactating mothers.
PNV Tabs 20-1 can also be beneficial in improving the nutritional status of women prior to conception.
- CONTRAINDICATIONS
- PRECAUTIONS
- WARNINGS
-
ADVERSE REACTIONS
Allergic sensitization has been reported following both oral and parenteral administration of folic acid.
You should call your doctor for medical advice about serious adverse events. To report adverse side effects or to obtain product information, contact Redmont Pharmaceuticals, LLC at 1-800-986-5909.
- DOSAGE AND ADMINISTRATION
-
HOW SUPPLIED
Bottles of 30 tablets (71741-091-301). Tablet is light blue, oblong.
This product is a prescription-folate with or without other dietary ingredients that – due to increased folate levels increased risk associated with masking B12 deficiency (pernicious anemia) requires administration under the care of a licensed medical practitioner (64 FR 8760). 1-3 The most appropriate way to ensure pedigree reporting consistent with these regulatory guidelines and safety monitoring is to dispense this product only by prescription. This is not an Orange Book product. This product may be administered only under a physician's supervision and all prescriptions using this product shall be pursuant to state statues as applicable. The ingredients, indication or claims of this product are not to be construed to be drug claims.
- Federal Register Notice of August 2, 1973
(38 FR 20750) - Federal Register Notice of October 17, 1980
(45 FR 69043, 69044) - Federal Register Notice of March 5, 1996
(61 FR 8760)
- 1
- Redmont Pharmaceuticals does not represent this product code to be National Drug Code (NDC). Product codes are formatted according to standard industry practice, to meet the formatting requirement by pedigree reporting and supply-chain control including pharmacies.
- Federal Register Notice of August 2, 1973
- STORAGE AND HANDLING
- TAMPER EVIDENT
-
HEALTH CLAIM
MADE IN USA
REDMONT
PHARMACEUTICALSDistributed by:
Redmont Pharmaceuticals, LLC
Birmingham, AL 35209
800-986-5909Ferrochel™ is a trademark of Albion Laboratories, Inc.
These statements have not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure, or prevent any disease. KEEP THIS AND ALL MEDICATIONS OUT OF THE REACH OF CHILDREN
© 2021 Redmont Pharmaceuticals, LLC
091111-0121 - PRINCIPAL DISPLAY PANEL - 30 Tablet Bottle Label
-
INGREDIENTS AND APPEARANCE
PNV TABS 20-1
beta carotene, ascorbic acid, cholecalciferol, .alpha.-tocopherol acetate, pyridoxine hydrochloride, biotin, folic acid, levomefolate calcium, cyanocobalamin, calcium carbonate, magnesium oxide, ferrous bisglycinate, and potassium iodide tabletProduct Information Product Type DIETARY SUPPLEMENT Item Code (Source) NHRIC:71741-091 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Beta Carotene (UNII: 01YAE03M7J) (Beta Carotene - UNII:01YAE03M7J) Beta Carotene 300 ug Ascorbic Acid (UNII: PQ6CK8PD0R) (Ascorbic Acid - UNII:PQ6CK8PD0R) Ascorbic Acid 60 mg Cholecalciferol (UNII: 1C6V77QF41) (Cholecalciferol - UNII:1C6V77QF41) Cholecalciferol 10 ug .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) (.ALPHA.-TOCOPHEROL - UNII:H4N855PNZ1) .ALPHA.-TOCOPHEROL ACETATE 4.5 mg PYRIDOXINE HYDROCHLORIDE (UNII: 68Y4CF58BV) (PYRIDOXINE - UNII:KV2JZ1BI6Z) PYRIDOXINE HYDROCHLORIDE 26 mg Biotin (UNII: 6SO6U10H04) (Biotin - UNII:6SO6U10H04) Biotin 0.28 mg Folic Acid (UNII: 935E97BOY8) (Folic Acid - UNII:935E97BOY8) Folic Acid 0.4 mg LEVOMEFOLATE CALCIUM (UNII: A9R10K3F2F) (LEVOMEFOLIC ACID - UNII:8S95DH25XC) LEVOMEFOLATE CALCIUM 0.6 mg Cyanocobalamin (UNII: P6YC3EG204) (Cyanocobalamin - UNII:P6YC3EG204) Cyanocobalamin 0.013 mg Calcium Carbonate (UNII: H0G9379FGK) (Calcium Cation - UNII:2M83C4R6ZB) Calcium Carbonate 80 mg Magnesium Oxide (UNII: 3A3U0GI71G) (Magnesium Cation - UNII:T6V3LHY838) Magnesium Oxide 25 mg FERROUS BISGLYCINATE (UNII: SFW1D987QV) (Ferrous Cation - UNII:GW89581OWR) Ferrous Cation 20 mg Potassium Iodide (UNII: 1C4QK22F9J) (Iodide Ion - UNII:09G4I6V86Q) Potassium Iodide 0.15 mg Inactive Ingredients Ingredient Name Strength MICROCRYSTALLINE CELLULOSE 102 (UNII: PNR0YF693Y) Maltodextrin (UNII: 7CVR7L4A2D) Croscarmellose Sodium (UNII: M28OL1HH48) Silicon Dioxide (UNII: ETJ7Z6XBU4) Stearic Acid (UNII: 4ELV7Z65AP) MAGNESIUM PALMITOSTEARATE (UNII: R4OXA9G5BV) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) Titanium Dioxide (UNII: 15FIX9V2JP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NHRIC:71741-091-30 30 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Dietary Supplement 01/19/2021 Supplement Facts Serving Size : Serving per Container : Amount Per Serving % Daily Value color scoring 1 shape size (solid drugs) 19 mm Labeler - Redmont Pharmaceuticals, LLC (080843607)