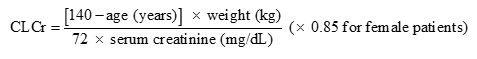Label: GABAPENTIN tablet, film coated
-
Contains inactivated NDC Code(s)
NDC Code(s): 71717-102-10, 71717-102-50, 71717-103-10, 71717-103-50 - Packager: Megalith Pharmaceuticals Inc
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated February 28, 2018
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use GABAPENTIN TABLETS safely and effectively. See full prescribing information for GABAPENTIN TABLETS. GABAPENTIN tablets, for ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEGabapentin is indicated for: Adjunctive therapy in the treatment of partial onset seizures, with and without secondary generalization, in adults and pediatric patients 3 years and older with ...
-
2 DOSAGE AND ADMINISTRATION2.2 Dosage for Epilepsy with Partial Onset Seizures - Patients 12 years of age and above - The starting dose is 300 mg three times a day. The recommended maintenance dose of gabapentin is 300 mg ...
-
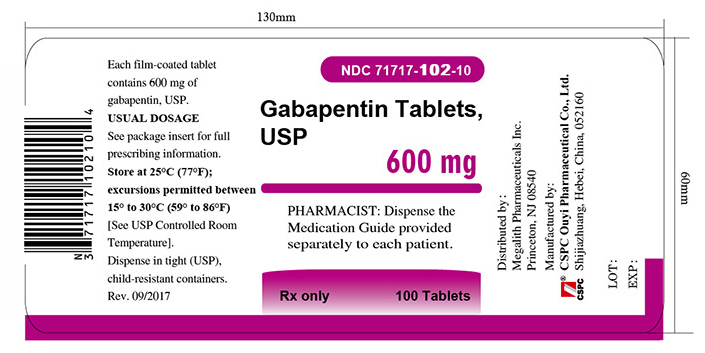
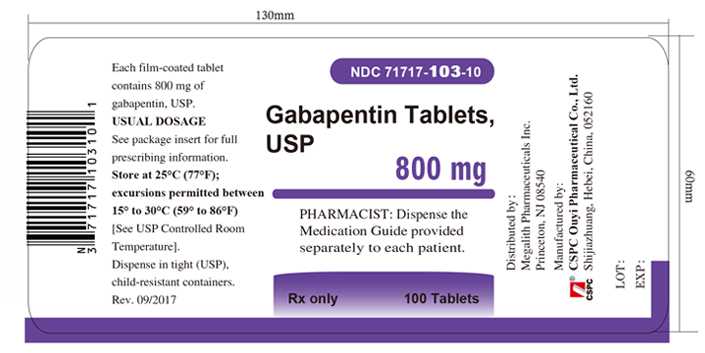
3 DOSAGE FORMS AND STRENGTHSGabapentin Tablets USP, 600 mg are white, elliptical, film-coated scored tablets debossed “O|E” on one side and “600” on the other side - Gabapentin Tablets USP, 800 mg are white, elliptical ...
-
4 CONTRAINDICATIONSGabapentin is contraindicated in patients who have demonstrated hypersensitivity to the drug or its ingredients.
-
5 WARNINGS AND PRECAUTIONS5.1 Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS)/Multiorgan Hypersensitivity - Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS), also known as multiorgan ...
-
6 ADVERSE REACTIONSThe following serious adverse reactions are discussed in greater detail in other sections: Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS)/Multiorgan Hypersensitivity ...
-
7 DRUG INTERACTIONS7.1 Other Antiepileptic Drugs - Gabapentin is not appreciably metabolized nor does it interfere with the metabolism of commonly coadministered antiepileptic drugs - [see Clinical ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Pregnancy Exposure Registry - There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to antiepileptic drugs (AEDs), such as gabapentin, during ...
-
9 DRUG ABUSE AND DEPENDENCE9.1 Controlled Substance - Gabapentin is not a scheduled drug. 9.2 Abuse - Gabapentin does not exhibit affinity for benzodiazepine, opiate (mu, delta or kappa), or cannabinoid 1 receptor sites ...
-
10 OVERDOSAGEA lethal dose of gabapentin was not identified in mice and rats receiving single oral doses as high as 8,000 mg/kg. Signs of acute toxicity in animals included ataxia, labored breathing, ptosis ...
-
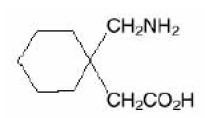
11 DESCRIPTIONThe active ingredient in gabapentin tablets, USP is gabapentin, which has the chemical name 1-(aminomethyl)cyclohexaneacetic acid. The molecular formula of gabapentin is C - 9H ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - The precise mechanism by which gabapentin produces its antiepileptic action is unknown. Gabapentin is structurally related to the neurotransmitter gamma-aminobutyric ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - Gabapentin was administered orally to mice and rats in 2-year carcinogenicity studies. No evidence of drug-related ...
-
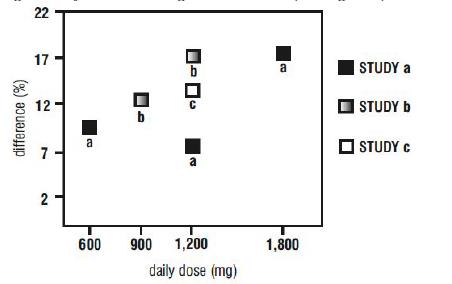
14 CLINICAL STUDIES14.2 Epilepsy for Partial Onset Seizures (Adjunctive Therapy) The effectiveness of gabapentin as adjunctive therapy (added to other antiepileptic drugs) was established in multicenter ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGGabapentin tablets, USP are supplied as follows: 600 mg tablets: White, elliptical, film-coated scored tablets debossed “O|E” on one side and “600” on the other side; available in: Bottles of 100 ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Medication Guide). Administration Information - Inform patients that gabapentin is taken orally with or without food. Inform patients ...
-
MEDICATION GUIDE
Gabapentin Tablets, USP
(GA-ba-PEN-tin)
MEDICATION GUIDE - Gabapentin Tablets, USP - (GA-ba-PEN-tin) What is the most important information I should know about gabapentin tablets? Do not stop taking gabapentin tablets without first ...
-
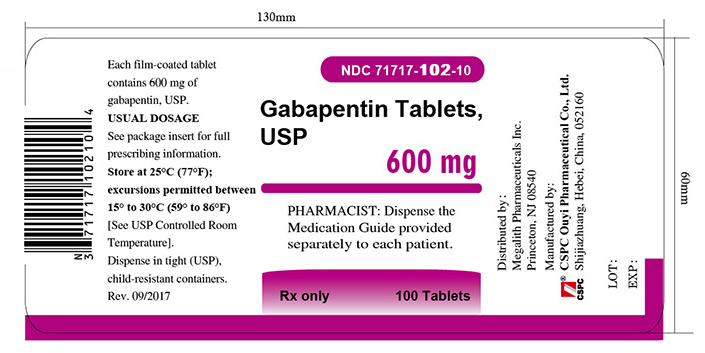
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - Tablet - 600 mgALWAYS DISPENSE WITH MEDICATION GUIDE - NDC 71717-102-10 - Gabapentin Tablets, USP - 600 mg - Rx only - 100 Tablets - CSPC - NDC 71717-102-50 - Gabapentin Tablets ...
-
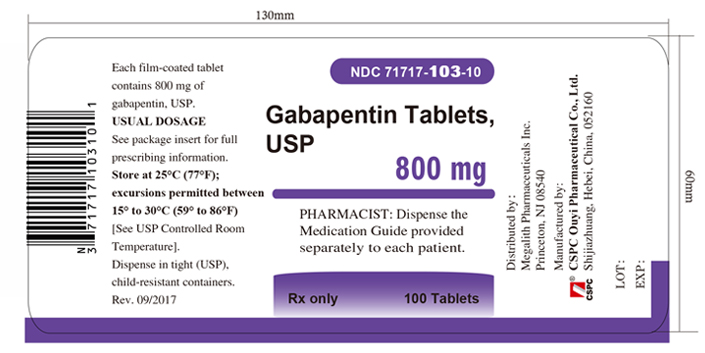
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - Tablet - 800 mgALWAYS DISPENSE WITH MEDICATION GUIDE - NDC 71717-103-10 - Gabapentin Tablets, USP - 800 mg - Rx only - 100 Tablets - CSP - NDC 71717-103-50 - Gabapentin Tablets ...
-
INGREDIENTS AND APPEARANCEProduct Information