Label: PENTOXIFYLLINE tablet, extended release
- NDC Code(s): 70954-668-10, 70954-668-20
- Packager: ANI Pharmaceuticals, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated January 15, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
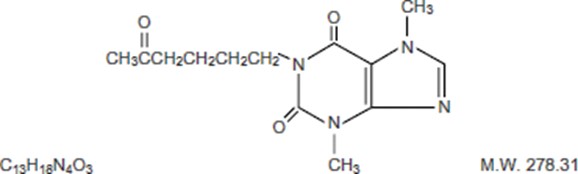
Pentoxifylline Extended-Release Tablets USP for oral administration contain 400 mg of the active drug and the following inactive ingredients: hypromellose, povidone, talc, magnesium stearate ...
-
CLINICAL PHARMACOLOGY Mode of Action - Pentoxifylline and its metabolites improve the flow properties of blood by decreasing its viscosity. In patients with chronic peripheral arterial disease, this increases blood ...
-
INDICATIONS AND USAGE Pentoxifylline Extended-Release Tablets are indicated for the treatment of patients with intermittent claudication on the basis of chronic occlusive arterial disease of the limbs. Pentoxifylline ...
-
CONTRAINDICATIONS Pentoxifylline Extended-Release Tablets should not be used in patients with recent cerebral and/or retinal hemorrhage or in patients who have previously exhibited intolerance to this product or ...
-
PRECAUTIONS General - At the first sign of anaphylactic/anaphylactoid reaction, Pentoxifylline Extended-Release Tablets must be discontinued. Patients with chronic occlusive arterial disease of the limbs ...
-
ADVERSE REACTIONS Clinical trials were conducted using either extended-release pentoxifylline tablets for up to 60 weeks or immediate-release pentoxifylline capsules for up to 24 weeks. Dosage ranges in the tablet ...
-
OVERDOSAGE Overdosage with pentoxifylline has been reported in pediatric patients and adults. Symptoms appear to be dose related. A report from a poison control center on 44 patients taking overdoses of ...
-
DOSAGE AND ADMINISTRATION The usual dosage of pentoxifylline in extended-release tablet form is one tablet (400 mg) three times a day with meals. While the effect of pentoxifylline may be seen within 2 to 4 weeks, it is ...
-
HOW SUPPLIED Pentoxifylline Extended-Release Tablets USP, 400 mg are available for oral administration as yellow, oblong, film-coated tablets, embossed with “N668” on one side and plain on the other. They are ...
-
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL NDC 70954-668-10 - Pentoxifylline Extended-Release Tablets USP - 400 mg - Rx only - 100 Tablets
-
INGREDIENTS AND APPEARANCEProduct Information