Label: PYRAZINAMIDE tablet
- NDC Code(s): 70954-484-10, 70954-484-20, 70954-484-30, 70954-484-40
- Packager: Novitium Pharma LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated May 12, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
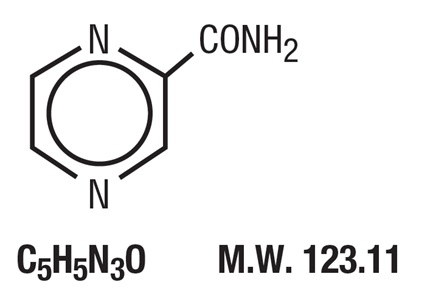
DESCRIPTIONPyrazinamide, the pyrazine analogue of nicotinamide, is an antituberculous agent. It is a white crystalline powder, stable at room temperature, and sparingly soluble in water. Pyrazinamide has the ...
-
CLINICAL PHARMACOLOGYPyrazinamide is well absorbed from the GI tract and attains peak plasma concentrations within 2 hours. Plasma concentrations generally range from 30 to 50 mcg/mL with doses of 20 to 25 mg/kg. It ...
-
INDICATIONS & USAGEPyrazinamide is indicated for the initial treatment of active tuberculosis in adults and children when combined with other antituberculous agents. (The current recommendation of the CDC for ...
-
CONTRAINDICATIONSPyrazinamide is contraindicated in persons: with severe hepatic damage. who have shown hypersensitivity to it. with acute gout.
-
WARNINGSPatients started on pyrazinamide should have baseline serum uric acid and liver function determinations. Those patients with preexisting liver disease or those at increased risk for drug related ...
-
PRECAUTIONSGENERAL PRECAUTIONS - Pyrazinamide inhibits renal excretion of urates, frequently resulting in hyperuricemia which is usually asymptomatic. If hyperuricemia is accompanied by acute gouty ...
-
ADVERSE REACTIONSGeneral - Fever, porphyria and dysuria have rarely been reported. Gout (see PRECAUTIONS). Gastrointestinal - The principal adverse effect is a hepatic reaction (see WARNINGS). Hepatotoxicity ...
-
OVERDOSAGEOverdosage experience is limited. In one case report of overdose, abnormal liver function tests developed. These spontaneously reverted to normal when the drug was stopped. Clinical monitoring and ...
-
DOSAGE & ADMINISTRATIONPyrazinamide should always be administered with other effective antituberculous drugs. It is administered for the initial 2 months of a 6-month or longer treatment regimen for drug-susceptible ...
-
HOW SUPPLIEDPyrazinamide Tablets, USP 500 mg are round, white, scored tablets, debossed "N" above the score and "484" below the score. NDC 70954-484-10 - Bottle of 60 - NDC 70954-484-20 - Bottle of 90 - NDC ...
-
REFERENCESDrug Information, American Hospital Formulary Service. American Society of Hospital Pharmacists. Bethesda, MD. 1991. USPDI, Drug Information for the Health Care Professional. United States ...
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANELPyrazinamide Tablets, USP 500 mg - 70954-484-10 - Bottle of 60 - Pyrazinamide Tablets, USP 500 mg - 70954-484-20 - Bottle of 90 - Pyrazinamide Tablets, USP 500 mg - 70954-484-30 - Bottle of ...
-
INGREDIENTS AND APPEARANCEProduct Information