Label: TOPROL XL- metoprolol succinate tablet, extended release
- NDC Code(s): 70842-110-02, 70842-111-02, 70842-112-02, 70842-113-02
- Packager: Melinta Therapeutics, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated November 28, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use TOPROL-XL® safely and effectively. See full prescribing information for TOPROL-XL. TOPROL-XL® (metoprolol succinate) Tablet ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE1.1 Hypertension - TOPROL-XL is indicated for the treatment of hypertension, to lower blood pressure. Lowering blood pressure lowers the risk of fatal and non-fatal cardiovascular events ...
-
2 DOSAGE AND ADMINISTRATION2.1 Hypertension - Adults: The usual initial dosage is 25 to 100 mg daily in a single dose. Adjust dosage at weekly (or longer) intervals until optimum blood pressure reduction is achieved ...
-
3 DOSAGE FORMS AND STRENGTHS25 mg tablets: White, oval, biconvex, film-coated scored tablet engraved with "A/β". 50 mg tablets: White, round, biconvex, film-coated scored tablet engraved with "A/mo". 100 mg tablets: White ...
-
4 CONTRAINDICATIONSTOPROL-XL is contraindicated in severe bradycardia, second- or third-degree heart block, cardiogenic shock, decompensated heart failure, sick sinus syndrome (unless a permanent pacemaker is in ...
-
5 WARNINGS AND PRECAUTIONS5.1 Abrupt Cessation of Therapy - Following abrupt cessation of therapy with certain beta-blocking agents, exacerbations of angina pectoris and, in some cases, myocardial infarction have ...
-
6 ADVERSE REACTIONSThe following adverse reactions are described elsewhere in labeling: Worsening angina or myocardial infarction [see Warnings and Precautions (5)] Worsening heart failure [see Warnings and ...
-
7 DRUG INTERACTIONS7.1 Catecholamine Depleting Drugs - Catecholamine depleting drugs (e.g., reserpine, monoamine oxidase (MAO) inhibitors) may have an additive effect when given with beta-blocking agents. Observe ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Untreated hypertension and heart failure during pregnancy can lead to adverse outcomes for the mother and the fetus (see Clinical Considerations). Available data ...
-
10 OVERDOSAGESigns and Symptoms - Overdosage of TOPROL-XL may lead to severe bradycardia, hypotension, and cardiogenic shock. Clinical presentation can also include atrioventricular block, heart failure ...
-
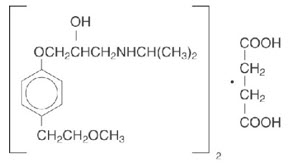
11 DESCRIPTIONTOPROL-XL, metoprolol succinate, is a beta1-selective (cardioselective) adrenoceptor blocking agent, for oral administration, available as extended-release tablets. TOPROL-XL has been formulated ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Metoprolol is a beta1-selective (cardioselective) adrenergic receptor blocking agent. This preferential effect is not absolute, however, and at higher plasma ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Long-term studies in animals have been conducted to evaluate the carcinogenic potential of metoprolol tartrate. In 2-year studies in ...
-
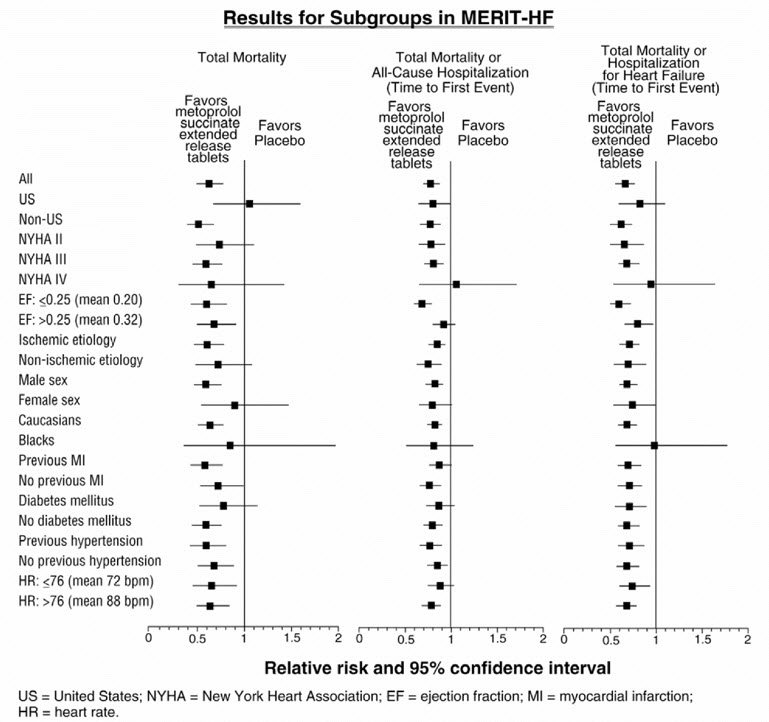
14 CLINICAL STUDIES14.1 Hypertension - In a double-blind study, 1092 patients with mild-to-moderate hypertension were randomized to once daily TOPROL-XL (25, 100, or 400 mg), PLENDIL® (felodipine extended-release ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGTablets containing metoprolol succinate equivalent to the indicated weight of metoprolol tartrate, USP, are white, biconvex, film-coated, and scored. TabletShapeEngravingBottle of 100 - NDC ...
-
17 PATIENT COUNSELING INFORMATIONAdvise patients to take TOPROL-XL regularly and continuously, as directed, preferably with or immediately following meals. If a dose is missed, the patient should take only the next scheduled dose ...
-
SPL UNCLASSIFIED SECTIONTOPROL-XL® and PLENDIL are trademarks of the AstraZeneca group of companies. Manufactured by: AstraZeneca AB - Södertälje, Swede - Product of Sweden - Distributed by: Melinta Therapeutics - Lincolnshire ...
-
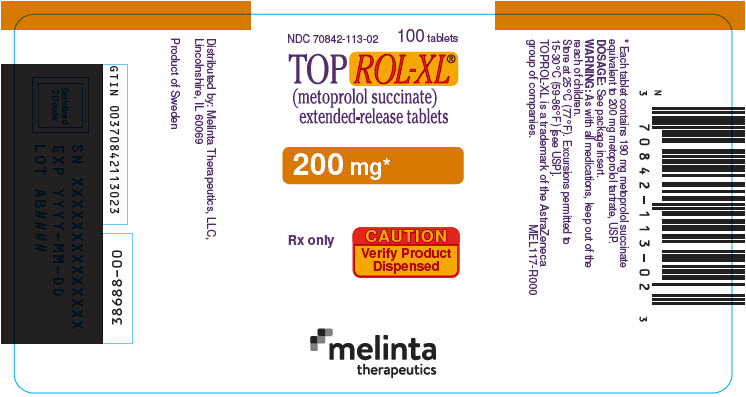
PRINCIPAL DISPLAY PANEL - 200 mg Tablet Bottle LabelNDC 70842-113-02 - 100 tablets - TOPROL-XL® (metoprolol succinate) extended-release tablets - 200 mg* Rx only - CAUTION - Verify Product - Dispensed - melinta - therapeutics
-
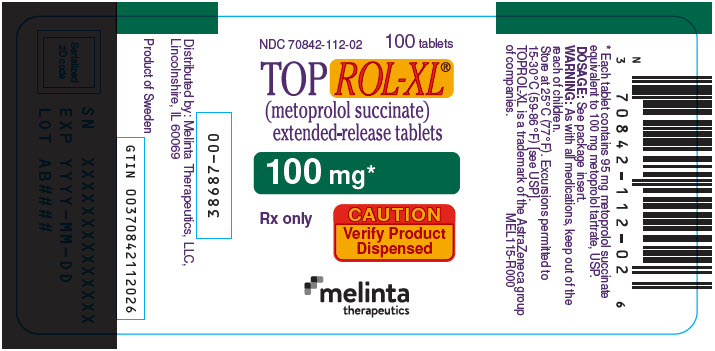
PRINCIPAL DISPLAY PANEL - 100 mg Tablet Bottle LabelNDC 70842-112-02 - 100 tablets - TOPROL-XL® (metoprolol succinate) extended-release tablets - 100 mg* Rx only - CAUTION - Verify Product - Dispensed - melinta - therapeutics
-
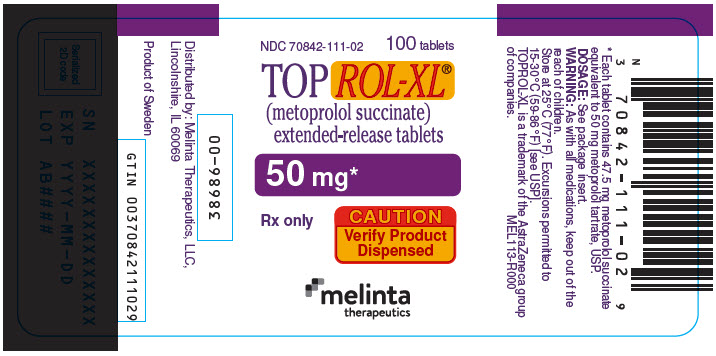
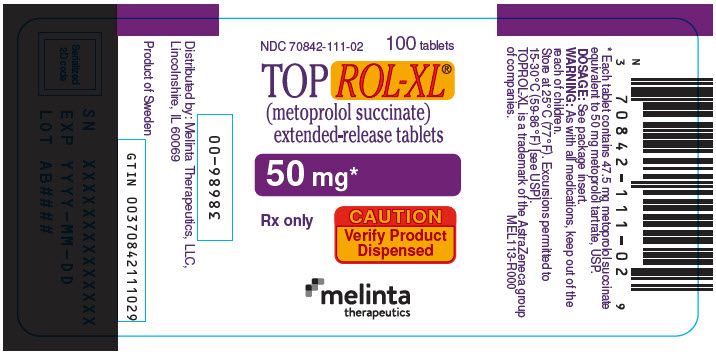
PRINCIPAL DISPLAY PANEL - 50 mg Tablet Bottle LabelNDC 70842-111-02 - 100 tablets - TOPROL-XL® (metoprolol succinate) extended-release tablets - 50 mg* Rx only - CAUTION - Verify Product - Dispensed - melinta - therapeutics
-
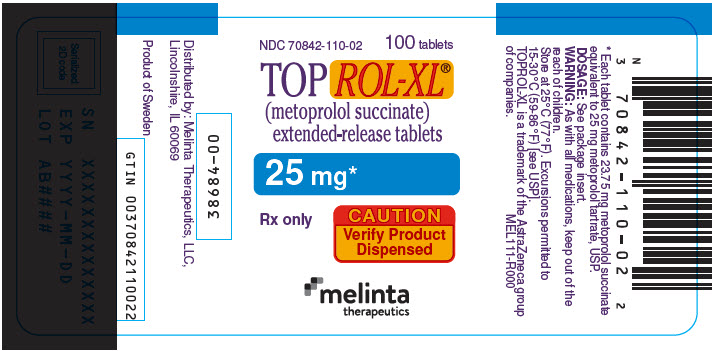
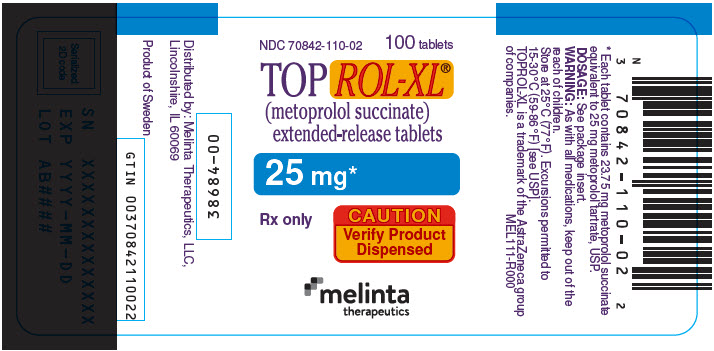
PRINCIPAL DISPLAY PANEL - 25 mg Tablet Bottle LabelNDC 70842-110-02 - 100 tablets - TOPROL-XL® (metoprolol succinate) extended-release tablets - 25 mg* Rx only - CAUTION - Verify Product - Dispensed - melinta - therapeutics
-
INGREDIENTS AND APPEARANCEProduct Information