Label: ALOSETRON tablet
- NDC Code(s): 70756-701-30, 70756-702-30
- Packager: Lifestar Pharma LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated March 23, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use ALOSETRON TABLETS safely and effectively. See full prescribing information for ALOSETRON TABLETS. ALOSETRON tablets, for oral use ...
-
Table of ContentsTable of Contents
-
BOXED WARNING
(What is this?)
WARNING: SERIOUS GASTROINTESTINAL ADVERSE REACTIONS
Infrequent but serious gastrointestinal adverse reactions have been reported with the use of alosetron tablets. These events, including ischemic colitis and serious complications of constipation, have resulted in hospitalization, and rarely, blood transfusion, surgery, and death.
- Alosetron tablets are indicated only for women with severe diarrhea-predominant irritable bowel syndrome (IBS) who have not responded adequately to conventional therapy [see Indications and Usage (1)] .
- Alosetron tablets should be discontinued immediately in patients who develop constipation or symptoms of ischemic colitis. Patients should immediately report constipation or symptoms of ischemic colitis to their prescriber. Alosetron tablets should not be resumed in patients who develop ischemic colitis. Patients who have constipation should immediately contact their prescriber if the constipation does not resolve after alosetron tablets is discontinued. Patients with resolved constipation should resume alosetron tablets only on the advice of their treating prescriber [see Dosage and Administration (2.1), Warnings and Precautions (5.1), (5.2)].
-
1 INDICATIONS AND USAGEAlosetron tablets are indicated only for women with severe diarrhea-predominant irritable bowel syndrome (IBS) who have: chronic IBS symptoms (generally lasting 6 months or longer), had ...
-
2 DOSAGE AND ADMINISTRATION2.1 Adult Patients - To lower the risk of constipation, alosetron tablets should be started at a dosage of 0.5 mg twice a day. Patients who become constipated at this dosage should stop taking ...
-
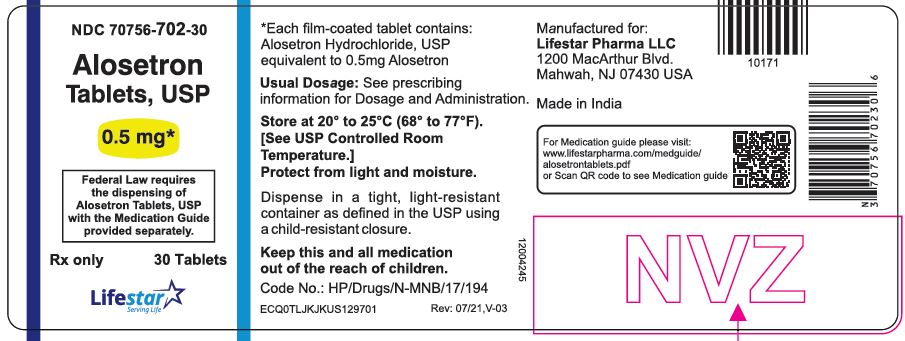
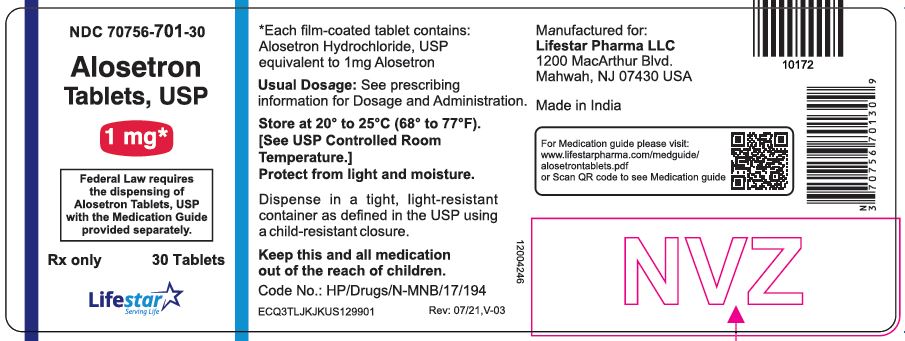
3 DOSAGE FORMS AND STRENGTHS0.5 mg and 1 mg tablets - Alosetron Tablets USP, 0.5 mg (0.562 mg alosetron HCl equivalent to 0.5 mg alosetron), are round, film-coated, white colored biconvex tablets debossed with "LS 702" on one ...
-
4 CONTRAINDICATIONS4.1 Constipation - Alosetron tablets should not be initiated in patients with constipation [see Warnings and Precautions (5.1)]. 4.2 History of Severe Bowel or Hepatic Disorders - Alosetron ...
-
5 WARNINGS AND PRECAUTIONS5.1 Serious Complications of Constipation - Some patients have experienced serious complications of constipation without warning. Serious complications of constipation, including ...
-
6 ADVERSE REACTIONSThe following adverse reactions are described in more detail in other sections of the label: Complications of constipation [see Boxed Warning, Warnings and Precautions (5.1)] Ischemic colitis ...
-
7 DRUG INTERACTIONSIn vivo data suggest that alosetron is primarily metabolized by cytochrome P450 (CYP) 1A2, with minor contributions from CYP3A4 and CYP2C9. Therefore, inducers or inhibitors of these enzymes may ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - The available data with alosetron tablets use in pregnant women are insufficient to draw conclusions about any drug-associated risks for major birth defects ...
-
10 OVERDOSAGEThere is no specific antidote for overdose of alosetron tablets. Patients should be managed with appropriate supportive therapy. Individual oral doses as large as 16 mg have been administered in ...
-
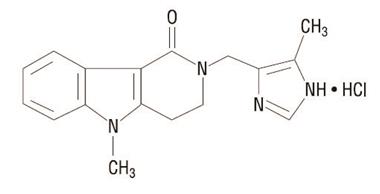
11 DESCRIPTIONThe active ingredient in alosetron tablets USP, is alosetron hydrochloride (HCl) USP, a potent and selective antagonist of the serotonin 5-HT3 receptor type. Chemically, alosetron is designated ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Alosetron is a potent and selective 5-HT3 receptor antagonist. 5-HT3 receptors are ligand-gated cation channels that are extensively distributed on enteric neurons ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - In 2-year oral studies, alosetron was not carcinogenic in mice at doses up to 30 mg/kg/day or in rats at doses up to 40 mg/kg/day ...
-
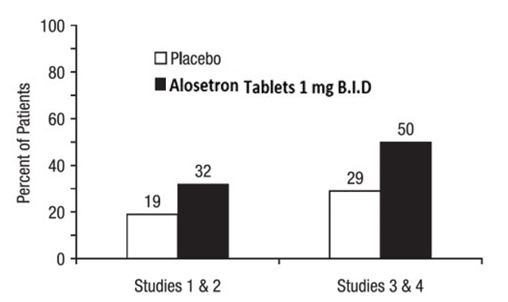
14 CLINICAL STUDIES14.1 Dose-Ranging Study - Data from a dose-ranging study of women (n = 85) who received alosetron tablets 0.5 mg twice daily indicated that the incidence of constipation (14%) was lower than ...
-
15 REFERENCES1. Thompson WG, Creed F, Drossman DA, et al. Functional bowel disease and functional abdominal pain. Gastroenterol Int. 1992;5:75-91.
-
16 HOW SUPPLIED/STORAGE AND HANDLINGAlosetron Tablets USP, 0.5 mg (0.562 mg alosetron HCl equivalent to 0.5 mg alosetron), are round, film coated, white colored biconvex tablets debossed with "LS 702" on one side and plain on other ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labelling (Medication Guide). Prescriber and Patient Responsibilities - Patients should be fully counseled on and understand the risks and ...
-
MEDICATION GUIDEMEDICATION GUIDE - Alosetron (a-LOE-se-tron) Tablets, USP - Read the Medication Guide you get with each refill for alosetron tablets. There may be new information. This Medication ...
-
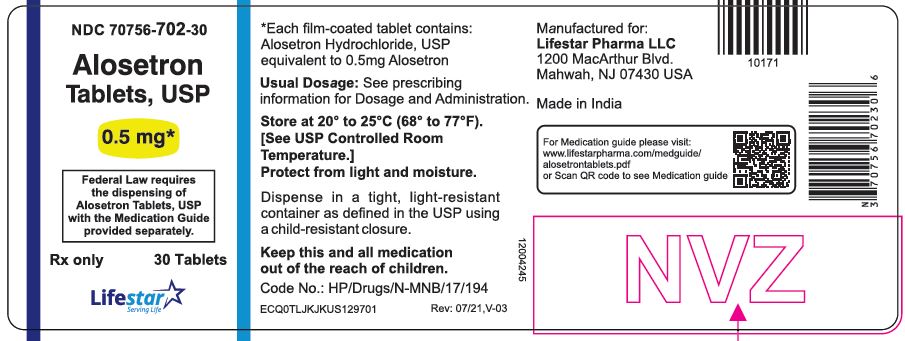
PACKAGE LABEL.PRINCIPAL DISPLAY PANELNDC 70756-702-30 - Alosetron Tablets, USP - 0.5 mg - Federal Law requires the dispensing of Alosetron Tablets, USP with the Medication Guide provided separately. Rx only - 30 Tablets
-
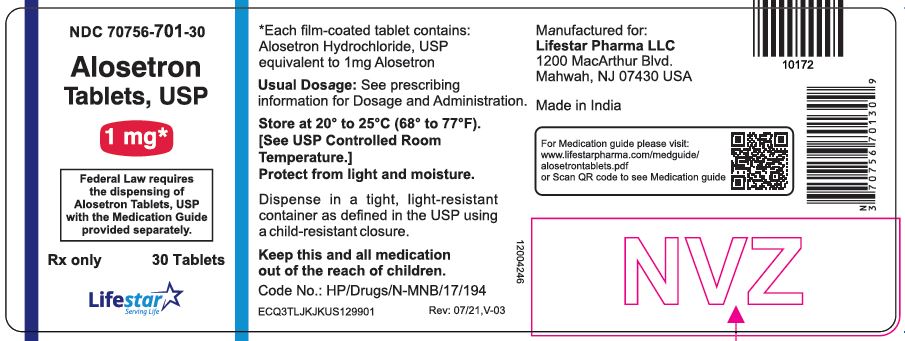
PACKAGE LABEL.PRINCIPAL DISPLAY PANELNDC 70756-701-30 - Alosetron Tablets, USP - 1 mg - Federal Law requires the dispensing of Alosetron Tablets, USP with the Medication Guide provided separately. Rx only - 30 Tablets
-
INGREDIENTS AND APPEARANCEProduct Information