Label: ATROPINE SULFATE solution/ drops
- NDC Code(s): 70756-651-25, 70756-652-25, 70756-653-35
- Packager: Lifestar Pharma LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated January 11, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use ATROPINE SULFATE OPHTHALMIC SOLUTION safely and effectively. See full prescribing information for ATROPINE SULFATE OPHTHALMIC SOLUTION.
ATROPINE SULFATE ophthalmic solution, for topical application to the eye.
Initial U.S. Approval: 1960INDICATIONS AND USAGE
Atropine is an anti-muscarinic agent indicated for: (1)
- Cycloplegia (1.1)
- Mydriasis (1.2)
- Penalization of the healthy eye in the treatment of amblyopia (1.3)
DOSAGE AND ADMINISTRATION
- In individuals from three (3) months of age or greater, 1 drop topically to the cul-de-sac of the conjunctiva, forty minutes prior to the intended maximal dilation time (2)
- In individuals 3 years of age or greater, doses may be repeated up to twice daily as needed. (2)
DOSAGE FORMS AND STRENGTHS
1% ophthalmic solution (3) (3)
CONTRAINDICATIONS
Hypersensitivity or allergic reaction to any ingredient in formulation (4.1) (4)
WARNINGS AND PRECAUTIONS
- Photophobia and blurred vision due to pupil unresponsiveness and cycloplegia may last up to 2 weeks. (5.1)
- Risk of blood pressure increase from systemic absorption (5.2)
ADVERSE REACTIONS
Most common adverse reactions that have been reported are eye pain and stinging on administration, blurred vision, photophobia, decreased lacrimation, increased heart rate and blood pressure (6) (6)
To report SUSPECTED ADVERSE REACTIONS, contact Lifestar Pharma LLC at 1-888-995-4337 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. (6)
DRUG INTERACTIONS
The use of atropine and monoamine oxidase inhibitors (MAOI) is generally not recommended because of the potential to precipitate hypertensive crisis. (7) (7)
USE IN SPECIFIC POPULATIONS
Should only be used in pregnant women if clearly needed (8) (8)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 8/2023
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
4.1 Hypersensitivity to any Component of this Medication
5 WARNINGS AND PRECAUTIONS
5.1 Photophobia and Blurred Vision
5.2 Elevation of Blood Pressure
6 ADVERSE REACTIONS
6.1 Ocular Adverse Reactions
6.2 Systemic Adverse Reactions
7 DRUG INTERACTIONS
7.1 Monamine oxidase inhibitors
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
- 2 DOSAGE AND ADMINISTRATION
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
- 5 WARNINGS AND PRECAUTIONS
-
6 ADVERSE REACTIONS
The following serious adverse reactions are described below and elsewhere in the labeling:
- Photophobia and Blurred Vision [See Warnings and Precautions (5.1)]
- Elevation in Blood Pressure [See Warnings and Precautions (5.2)]
The following adverse reactions have been identified following use of atropine sulfate ophthalmic solution. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
6.1 Ocular Adverse Reactions
Eye pain and stinging occurs upon instillation of atropine sulfate ophthalmic solution. Other commonly occurring adverse reactions include, blurred vision, photophobia, superficial keratitis and decreased lacrimation. Allergic reactions such as papillary conjunctivitis, contact dermatitis, and lid edema may also occur less commonly.
6.2 Systemic Adverse Reactions
Systemic effects of atropine are related to its anti-muscarinic activity. Systemic adverse events reported include dryness of skin, mouth, and throat from decreased secretions from mucus membranes; restlessness, irritability or delirium from stimulation of the central nervous system; tachycardia; flushed skin of the face and neck.
- 7 DRUG INTERACTIONS
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
There are no adequate and well-controlled studies of atropine sulfate in pregnant women. Animal development and reproduction studies have not been conducted with atropine sulfate. Since it is not known whether topically administered atropine sulfate can cause fetal harm, atropine sulfate ophthalmic solution, 1% should only be used during pregnancy if the potential benefit justifies the potential risk to the fetus.
8.3 Nursing Mothers
Traces of atropine have been found in human milk following administration of atropine solution for injection. Because some systemic absorption occurs from topical administration, caution should be exercised when Atropine Sulfate Ophthalmic Solution 1% is administered to a nursing woman.
8.4 Pediatric Use
Due to the potential for systemic absorption of atropine sulfate ophthalmic solution, the use of atropine sulfate ophthalmic solution, 1% in children under the age of 3 months is not recommended and the use in children under 3 years of age should be limited to no more than one drop per eye per day.
-
10 OVERDOSAGE
In the event of accidental ingestion or toxic overdosage with atropine sulfate ophthalmic solution supportive care may include a short acting barbiturate or diazepam as needed to control marked excitement and convulsions. Large doses for sedation should be avoided because central depressant action may coincide with the depression occurring late in atropine poisoning. Central stimulants are not recommended.
Physostigmine, given by slow intravenous injection of 1 to 4 mg (0.5 to 1 mg in pediatric populations), rapidly abolishes delirium and coma caused by large doses of atropine. Since physostigmine is rapidly destroyed, the patient may again lapse into coma after one to two hours, and repeated doses may be required.
Artificial respiration with oxygen may be necessary. Cooling measures may be needed to help to reduce fever, especially in pediatric populations.
The fatal adult dose of atropine is not known. In pediatric populations, 10 mg or less may be fatal.
-
11 DESCRIPTION
Atropine Sulfate Ophthalmic Solution, USP 1% is a sterile topical anticholinergic for ophthalmic use. The active ingredient is represented by the chemical structure
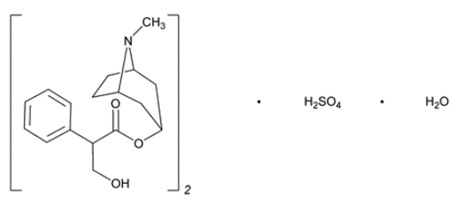
Chemical Name: Benzeneacetic acid, α-(hydroxymethyl)-, 8-methyl-8-azabicyclo[3.2.1.]oct-3-yl ester, endo –(±)-, sulfate (2:1) (salt), monohydrate.
Molecular Formula: (C17H23NO3)2 H2SO4 H2O
Molecular Weight: 694.84 g/mol
Atropine sulfate, USP appears as colorless, almost white to white solid. It is very soluble in water and glacial acetic acid, freely soluble in ethanol (96%) and practically insoluble in diethyl ether.
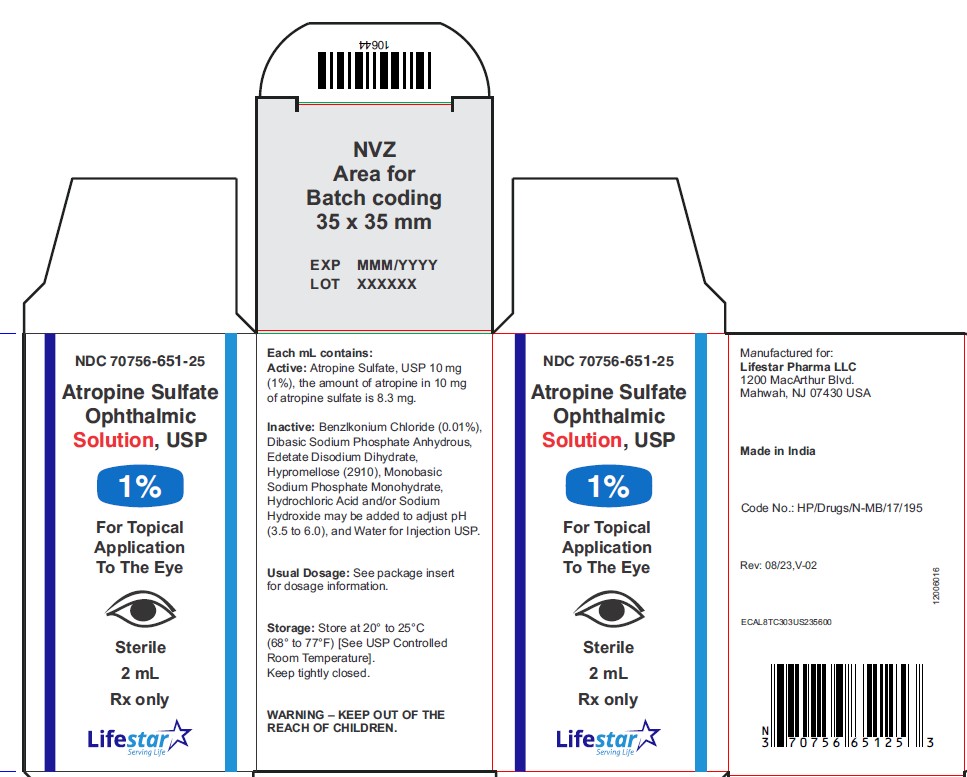
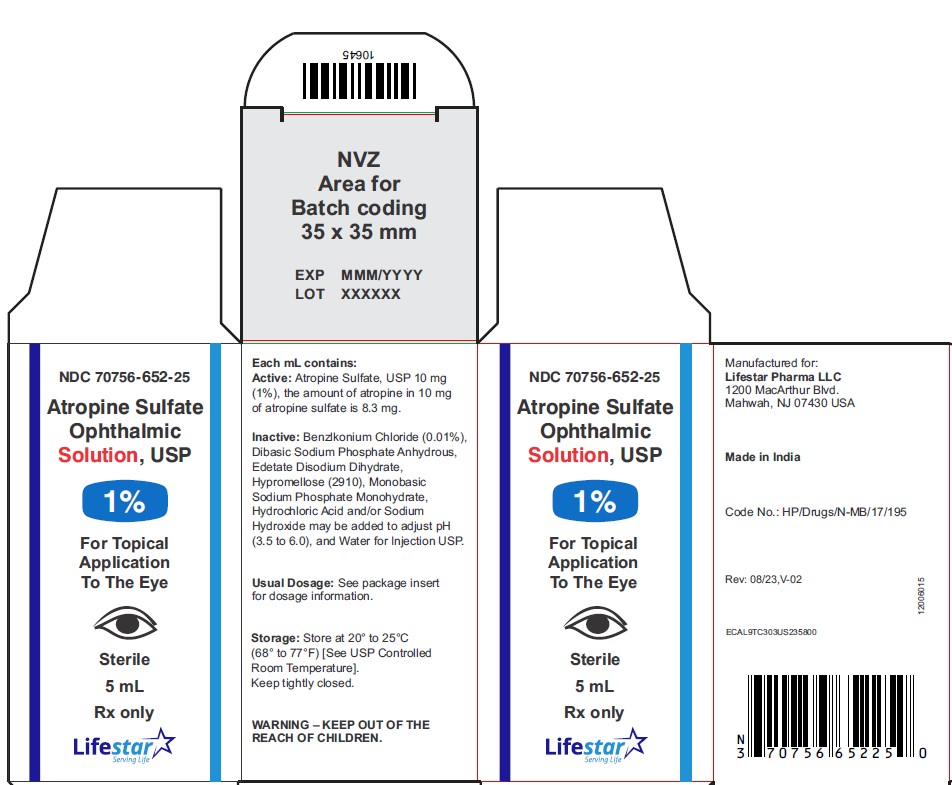
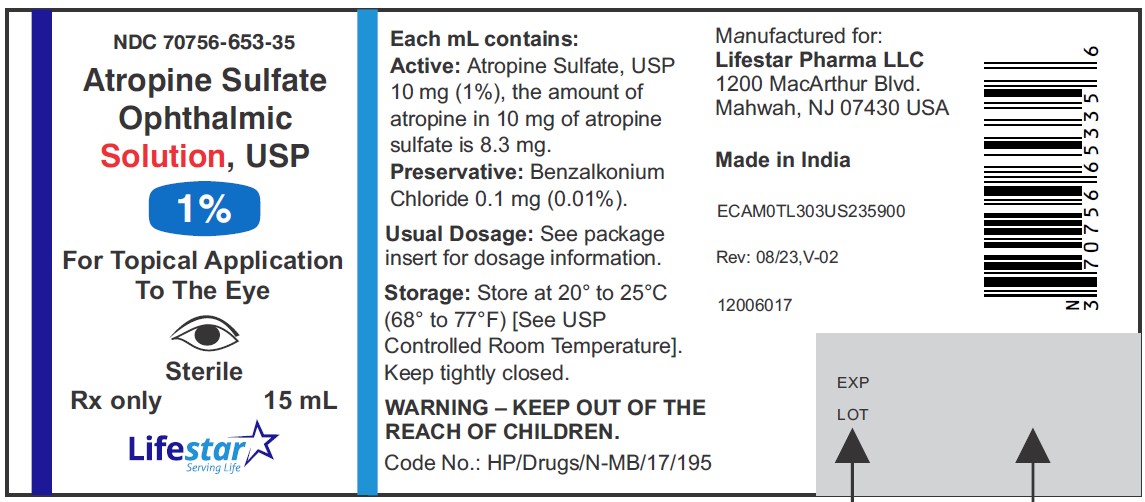
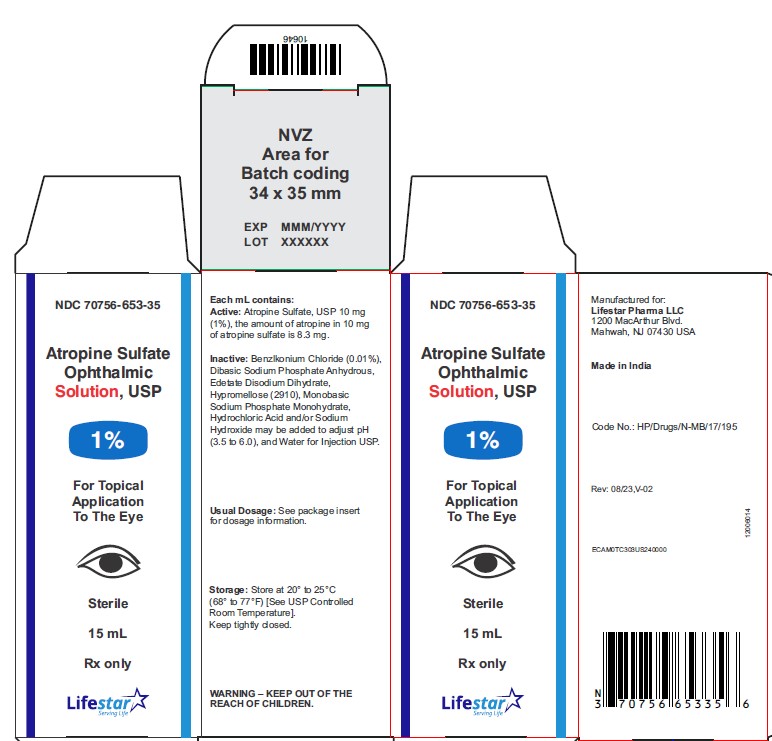
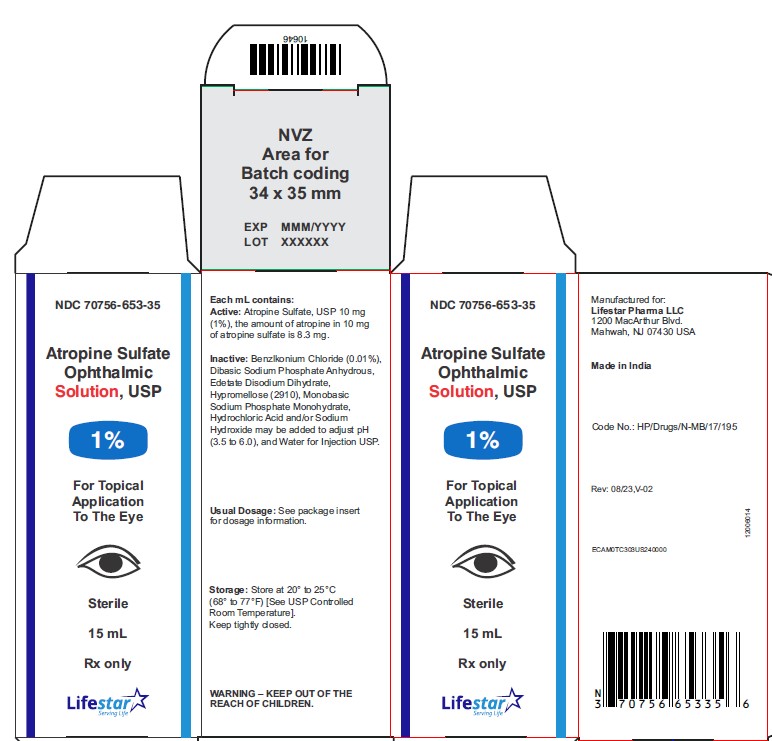
Each mL of Atropine Sulfate Ophthalmic Solution USP, 1% contains: Active: atropine sulfate, USP 10 mg equivalent to 8.3 mg of atropine. Inactives: benzalkonium chloride 0.1 mg (0.01%), dibasic sodium phosphate anhydrous, edetate disodium dihydrate, hypromellose (2910), monobasic sodium phosphate monohydrate, hydrochloric acid and/or sodium hydroxide may be added to adjust pH (3.5 to 6.0), and water for injection USP.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Atropine is a reversible antagonist of muscarine-like actions of acetyl-choline and is therefore classified as an antimuscarinic agent. Atropine is relatively selective for muscarinic receptors. Its potency at nicotinic receptors is much lower, and actions at non-muscarinic receptors are generally undetectable clinically. Atropine does not distinguish among the M1, M2, and M3 subgroups of muscarinic receptors.
The pupillary constrictor muscle depends on muscarinic cholinoceptor activation. This activation is blocked by topical atropine resulting in unopposed sympathetic dilator activity and mydriasis. Atropine also weakens the contraction of the ciliary muscle, or cycloplegia. Cycloplegia results in loss of the ability to accommodate such that the eye cannot focus for near vision.
12.2 Pharmacodynamics
The onset of action after administration of atropine sulfate ophthalmic solution 1%, is usually within 40 minutes with maximal effect being reached in about 2 hours. The effect can last for up to 2 weeks in a normal eye.
12.3 Pharmacokinetics
The bioavailability of atropine sulfate ophthalmic solution, 1% was assessed in six healthy subjects, 24 to 29 years of age. Subjects received either 0.3 mg atropine sulfate administered as bolus intravenous injection or 0.3 mg administered as 30 μl instilled unilaterally in the cul-de-sac of the eye. Plasma l-hyoscyamine concentrations were determined over selected intervals up to eight hours after dose administration.
The mean bioavailability of topically applied atropine was 63.5 ± 29% (range 19 to 95%) with large inter-individual differences. Mean maximum observed plasma concentration for the ophthalmic solution was 288 ± 73 pg/mL. Maximum concentration was reached in 28 ± 27 min after administration. Terminal half-life of l-hyoscamine was not affected by route of administration and was calculated to be 3 ± 1.2 hours (intravenous) and 2.5 ± 0.8 hours (topical ophthalmic).
In another placebo-controlled study, the systemic exposure to l-hyoscyamine, and the anti-cholinergic effects of atropine were investigated in eight ocular surgery patients 56 to 66 years of age, following single topical ocular 0.4 mg atropine dose (given as 40 microliters of atropine sulfate ophthalmic solution, 1%). The mean (± standard deviation (SD)) Cmax of l-hyoscyamine in these patients was 860 ± 402 pg/mL, achieved within 8 minutes of eyedrop instillation.
Following intravenous administration, the mean (± SD) elimination half-life (t1/2) of atropine was reported to be longer in pediatric subjects under 2 years (6.9 ± 3.3 hours) and in geriatric patients 65 to 75 years (10.0 ± 7.3 hours), compared to in children over 2 years (2.5 ± 1.2 hours) and in adults 16 to 58 years (3.0 ± 0.9 hours). (see 8.4 Pediatric Use).
Atropine is destroyed by enzymatic hydrolysis, particularly in the liver; from 13 to 50% is excreted unchanged in the urine. Traces are found in various secretions, including milk. The major metabolites of atropine are noratropine, atropin-n-oxide, tropine, and tropic acid. Atropine readily crosses the placental barrier and enters the fetal circulation, but is not found in amniotic fluid.
Atropine binds poorly (about 44%) to plasma protein, mainly to alpha-1 acid glycoprotein; age has no effect on the serum protein binding of atropine. Atropine binding to α-1 acid glycoprotein was concentration dependent (2 to 20 mcg/mL) and nonlinear in vitro and in vivo. There is no gender effect on the pharmacokinetics of atropine administered by injection.
- 13 NONCLINICAL TOXICOLOGY
-
14 CLINICAL STUDIES
Topical administration of atropine sulfate ophthalmic solution, 1% results in cycloplegia and mydriasis which has been demonstrated in several controlled clinical studies in adults and pediatric patients. Maximal mydriasis usually occurs in about 40 minutes and maximal cycloplegia is usually achieved in about 60 to 90 minutes after single administration. Full recovery usually occurs in approximately one week, but may last a couple of weeks.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Atropine Sulfate Ophthalmic Solution, USP 1% is supplied as clear, colorless solution in a three piece white bottle with a natural nozzle and red cap in the following sizes:
NDC 70756-651-25 2 mL fill in 5 mL bottle
NDC 70756-652-25 5 mL fill in 5 mL bottle
NDC 70756-653-35 15 mL fill in 15 mL bottle
Storage: Store at 20° to 25°C (68° to 77°F) [See USP Controlled Room Temperature]. Keep container tightly closed.
-
17 PATIENT COUNSELING INFORMATION
Advise patients not to touch the dropper tip to any surface as this may contaminate the solution.
Advise patients that drops will sting upon instillation and advise patients that they will experience sensitivity to light and blurred vision which may last for a couple of weeks.
Manufactured for:
Lifestar Pharma LLC
1200 MacArthur Blvd.
Mahwah, NJ 07430 USA
Made in India
Revised: 08/2023, V-02
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Atropine Sulfate Ophthalmic Solution, USP
1%
2 mL
Rx only
Sterile

Atropine Sulfate Ophthalmic Solution, USP
1%
2 mL
Rx only
Sterile
Atropine Sulfate Ophthalmic Solution, USP
1%
5 mL
Rx only
Sterile

Atropine Sulfate Ophthalmic Solution, USP
1%
5 mL
Rx only
Sterile
Atropine Sulfate Ophthalmic Solution, USP
1%
15 mL
Rx only
Sterile
Atropine Sulfate Ophthalmic Solution, USP
1%
15 mL
Rx only
Sterile
-
INGREDIENTS AND APPEARANCE
ATROPINE SULFATE
atropine sulfate solution/ dropsProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:70756-651 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ATROPINE SULFATE (UNII: 03J5ZE7KA5) (ATROPINE - UNII:7C0697DR9I) ATROPINE SULFATE 10 mg in 1 mL Inactive Ingredients Ingredient Name Strength HYPROMELLOSE 2910 (4000 MPA.S) (UNII: RN3152OP35) SODIUM PHOSPHATE, DIBASIC, ANHYDROUS (UNII: 22ADO53M6F) SODIUM PHOSPHATE, MONOBASIC, MONOHYDRATE (UNII: 593YOG76RN) EDETATE DISODIUM (UNII: 7FLD91C86K) BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) SODIUM HYDROXIDE (UNII: 55X04QC32I) HYDROCHLORIC ACID (UNII: QTT17582CB) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70756-651-25 1 in 1 CARTON 01/08/2024 1 2 mL in 1 BOTTLE; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA218148 01/08/2024 ATROPINE SULFATE
atropine sulfate solution/ dropsProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:70756-652 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ATROPINE SULFATE (UNII: 03J5ZE7KA5) (ATROPINE - UNII:7C0697DR9I) ATROPINE SULFATE 10 mg in 1 mL Inactive Ingredients Ingredient Name Strength HYPROMELLOSE 2910 (4000 MPA.S) (UNII: RN3152OP35) SODIUM PHOSPHATE, DIBASIC, ANHYDROUS (UNII: 22ADO53M6F) SODIUM PHOSPHATE, MONOBASIC, MONOHYDRATE (UNII: 593YOG76RN) EDETATE DISODIUM (UNII: 7FLD91C86K) BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) SODIUM HYDROXIDE (UNII: 55X04QC32I) HYDROCHLORIC ACID (UNII: QTT17582CB) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70756-652-25 1 in 1 CARTON 01/08/2024 1 5 mL in 1 BOTTLE; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA218148 01/08/2024 ATROPINE SULFATE
atropine sulfate solution/ dropsProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:70756-653 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ATROPINE SULFATE (UNII: 03J5ZE7KA5) (ATROPINE - UNII:7C0697DR9I) ATROPINE SULFATE 10 mg in 1 mL Inactive Ingredients Ingredient Name Strength HYPROMELLOSE 2910 (4000 MPA.S) (UNII: RN3152OP35) SODIUM PHOSPHATE, DIBASIC, ANHYDROUS (UNII: 22ADO53M6F) SODIUM PHOSPHATE, MONOBASIC, MONOHYDRATE (UNII: 593YOG76RN) EDETATE DISODIUM (UNII: 7FLD91C86K) BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) SODIUM HYDROXIDE (UNII: 55X04QC32I) HYDROCHLORIC ACID (UNII: QTT17582CB) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70756-653-35 1 in 1 CARTON 01/08/2024 1 15 mL in 1 BOTTLE; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA218148 01/08/2024 Labeler - Lifestar Pharma LLC (080268943) Registrant - Mankind Pharma Limited (915834068) Establishment Name Address ID/FEI Business Operations Mankind Pharma Limited 916512493 MANUFACTURE(70756-651, 70756-652, 70756-653) , ANALYSIS(70756-651, 70756-652, 70756-653) , PACK(70756-651, 70756-652, 70756-653)