Label: AMITRIPTYLINE HYDROCHLORIDE tablet, film coated
- NDC Code(s): 70756-201-11, 70756-201-12, 70756-202-11, 70756-202-12, view more
- Packager: Lifestar Pharma LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated April 9, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
- Rx only
-
BOXED WARNING
(What is this?)
Suicidality and Antidepressant Drugs
Antidepressants increased the risk compared to placebo of suicidal thinking and behavior (suicidality) in children, adolescents, and young adults in short-term studies of major depressive disorder (MDD) and other psychiatric disorders. Anyone considering the use of amitriptyline hydrochloride tablets or any other antidepressant in a child, adolescent, or young adult must balance this risk with the clinical need. Short-term studies did not show an increase in the risk of suicidality with antidepressants compared to placebo in adults beyond age 24; there was a reduction in risk with antidepressants compared to placebo in adults aged 65 and older. Depression and certain other psychiatric disorders are themselves associated with increases in the risk of suicide. Patients of all ages who are started on antidepressant therapy should be monitored appropriately and observed closely for clinical worsening, suicidality, or unusual changes in behavior. Families and caregivers should be advised of the need for close observation and communication with the prescriber. Amitriptyline hydrochloride tablets are not approved for use in pediatric patients (see WARNINGS: Clinical Worsening and Suicide Risk, PRECAUTIONS: Information for Patients, and PRECAUTIONS: Pediatric Use).
Close -
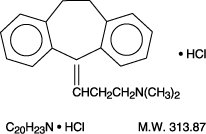
DESCRIPTIONAmitriptyline hydrochloride, USP, a dibenzocycloheptadiene derivative, is a white, or practically white, crystalline powder or small crystals which is freely soluble in water, in alcohol, in ...
-
CLINICAL PHARMACOLOGYAmitriptyline hydrochloride is an antidepressant with sedative effects. Its mechanism of action in man is not known. It is not a monoamine oxidase inhibitor and it does not act primarily by ...
-
INDICATIONS AND USAGEFor the relief of symptoms of depression. Endogenous depression is more likely to be alleviated than are other depressive states.
-
CONTRAINDICATIONSAmitriptyline hydrochloride is contraindicated in patients who have shown prior hypersensitivity to it. It should not be given concomitantly with monoamine oxidase inhibitors. Hyperpyretic ...
-
WARNINGSClinical Worsening and Suicide Risk - Patients with major depressive disorder (MDD), both adult and pediatric, may experience worsening of their depression and/or the emergence of suicidal ...
-
PRECAUTIONSSchizophrenic patients may develop increased symptoms of psychosis; patients with paranoid symptomatology may have an exaggeration of such symptoms. Depressed patients, particularly those with ...
-
ADVERSE REACTIONSWithin each category the following adverse reactions are listed in order of decreasing severity. Included in the listing are a few adverse reactions which have not been reported with this ...
-
OVERDOSAGEDeaths may occur from overdosage with this class of drugs. Multiple drug ingestion (including alcohol) is common in deliberate tricyclic antidepressant overdose. As the management is complex and ...
-
DOSAGE AND ADMINISTRATIONOral Dosage - Dosage should be initiated at a low level and increased gradually, noting carefully the clinical response and any evidence of intolerance. Initial Dosage for Adults - For ...
-
HOW SUPPLIEDAmitriptyline hydrochloride tablets, USP for oral administration are available as: 10 mg: Light pink to pink, round film coated tablet debossed with 'LS' on one side and '201' on other side and ...
-
METABOLISMStudies in man following oral administration of 14C-labeled drug indicated that amitriptyline is rapidly absorbed and metabolized. Radioactivity of the plasma was practically negligible, although ...
-
REFERENCESAyd FJ Jr: Amitriptyline therapy for depressive reactions. Psychosomatics 1960;1:320-325. Diamond S: Human metabolizer of amitriptyline tagged with carbon 14.Curr Ther Res, Mar 1965 ...
-
MEDICATION GUIDEDispense with Medication Guide available at: www.lifestarpharma.com/medguide/amitriptylinehcltablets.pdf - MEDICATION GUIDE - Amitriptyline Hydrochloride Tablets, USP - (a-mee-TRIP-ti-leen hye' ...
-
SPL UNCLASSIFIED SECTIONManufactured for: Lifestar Pharma LLC - 1200 MacArthur Blvd. Mahwah, NJ 07430 USA - Made in India - Revised: March 2025, V-03
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANELNDC 70756-201-11 - Amitriptyline Hydrochloride Tablets, USP - 10 mg - Rx Only - PHARMACIST: Dispense the Medication Guide provided separately to each patient. 100 Tablets
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANELNDC 70756-201-12 - Amitriptyline Hydrochloride Tablets, USP - 10 mg - Rx Only - PHARMACIST: Dispense the Medication Guide provided separately to each patient. 1000 Tablets
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANELNDC 70756-202-11 - Amitriptyline Hydrochloride Tablets, USP - 25 mg - Rx Only - PHARMACIST: Dispense the Medication Guide provided separately to each patient. 100 Tablets
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANELNDC 70756-202-12 - Amitriptyline Hydrochloride Tablets, USP - 25 mg - Rx Only - PHARMACIST: Dispense the Medication Guide provided separately to each patient. 1000 Tablets
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANELNDC 70756-203-11 - Amitriptyline Hydrochloride Tablets, USP - 50 mg - Rx Only - PHARMACIST: Dispense the Medication Guide provided separately to each patient. 100 Tablets
-
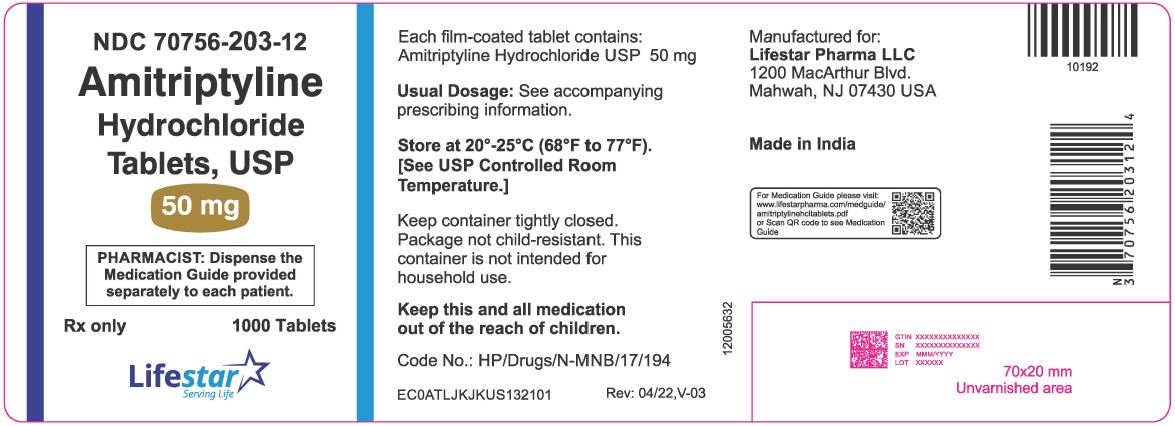
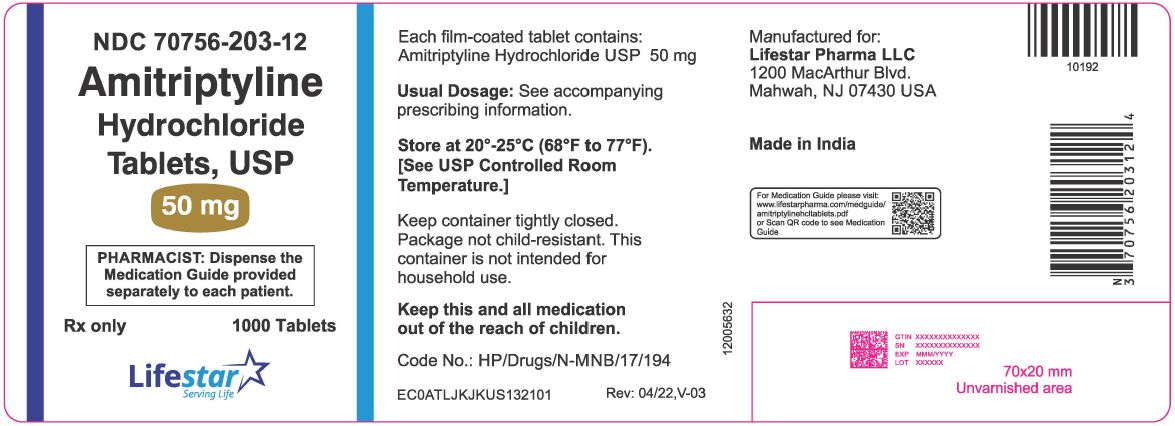
PACKAGE LABEL.PRINCIPAL DISPLAY PANELNDC 70756-203-12 - Amitriptyline Hydrochloride Tablets, USP - 50 mg - Rx Only - PHARMACIST: Dispense the Medication Guide provided separately to each patient. 1000 Tablets
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANELNDC 70756-204-11 - Amitriptyline Hydrochloride Tablets, USP - 75 mg - Rx Only - PHARMACIST: Dispense the Medication Guide provided separately to each patient. 100 Tablets
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANELNDC 70756-205-11 - Amitriptyline Hydrochloride Tablets, USP - 100 mg - Rx Only - PHARMACIST: Dispense the Medication Guide provided separately to each patient. 100 Tablets
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANELNDC 70756-206-11 - Amitriptyline Hydrochloride Tablets, USP - 150 mg - Rx Only - PHARMACIST: Dispense the Medication Guide provided separately to each patient. 100 Tablets
-
INGREDIENTS AND APPEARANCEProduct Information