Label: VARENICLINE tablet, film coated
VARENICLINE TARTRATE- varenicline tartrate kit
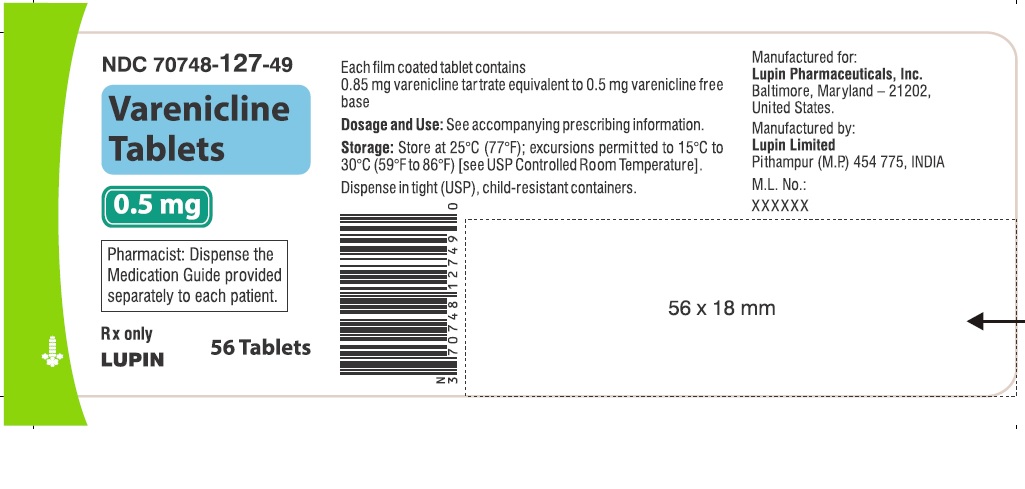
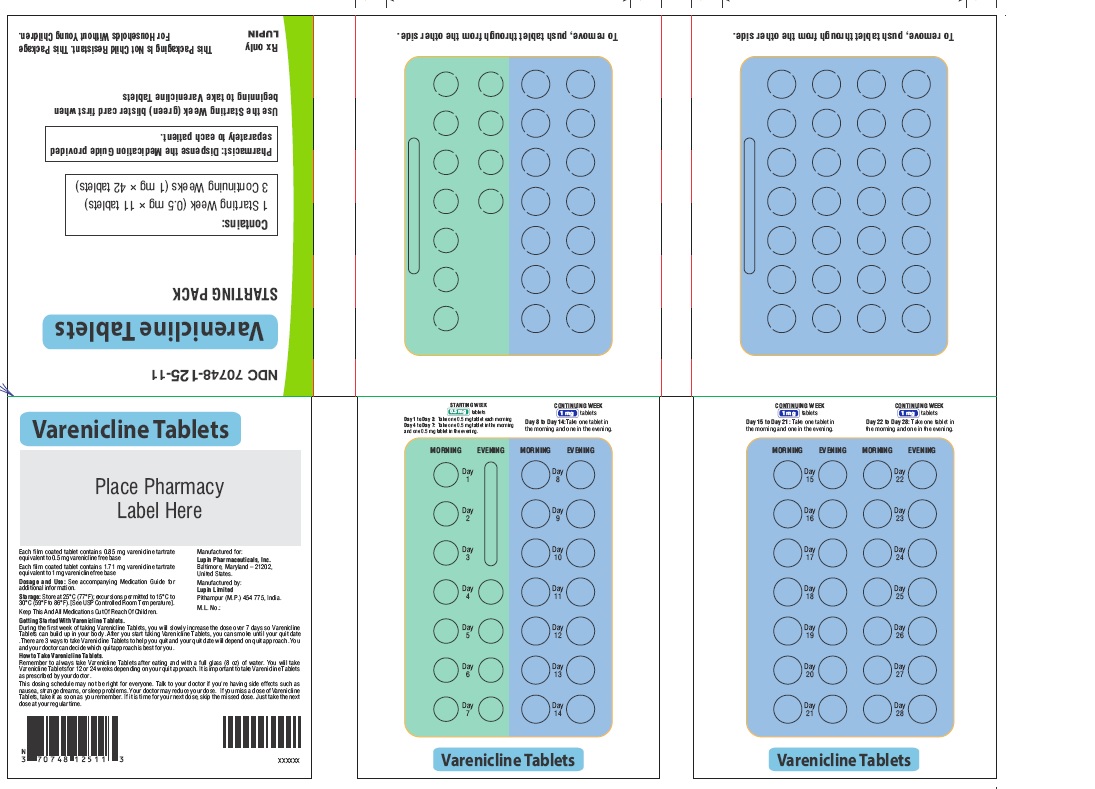
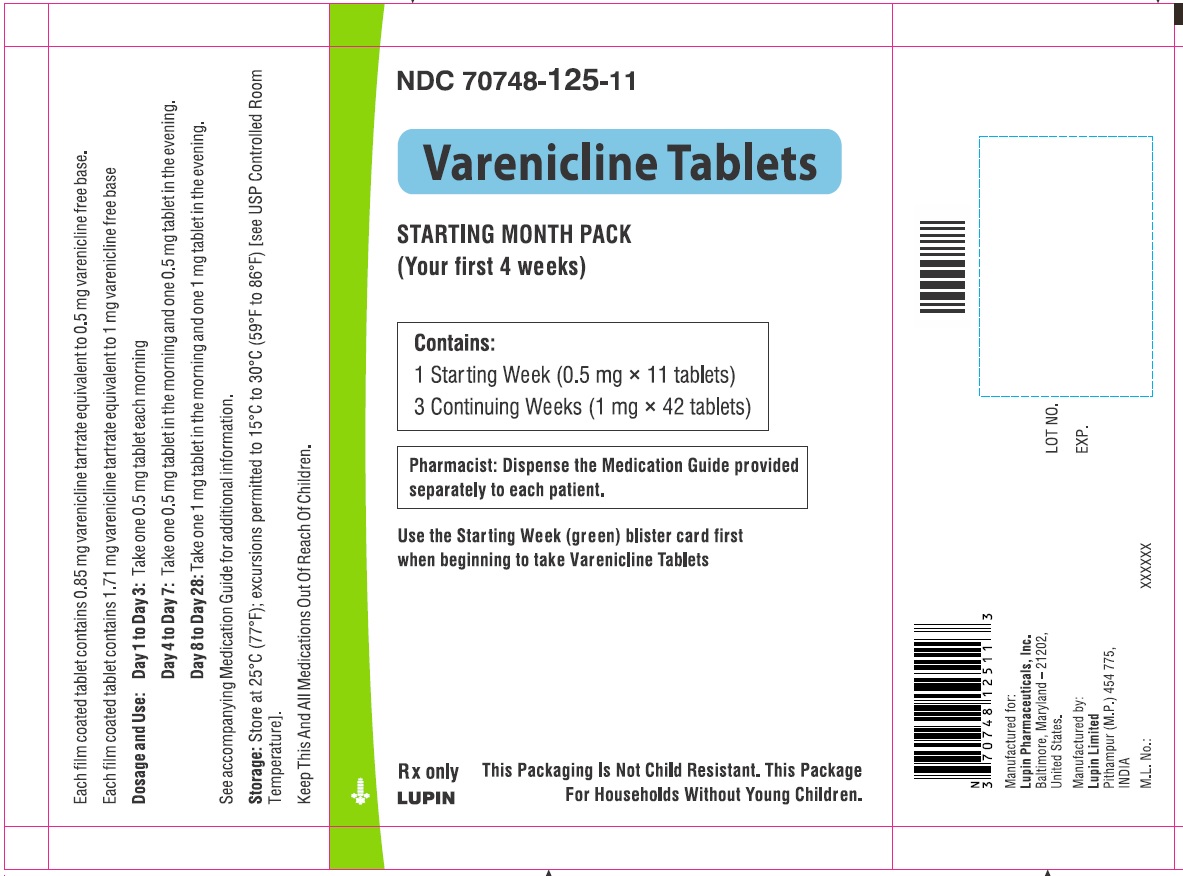
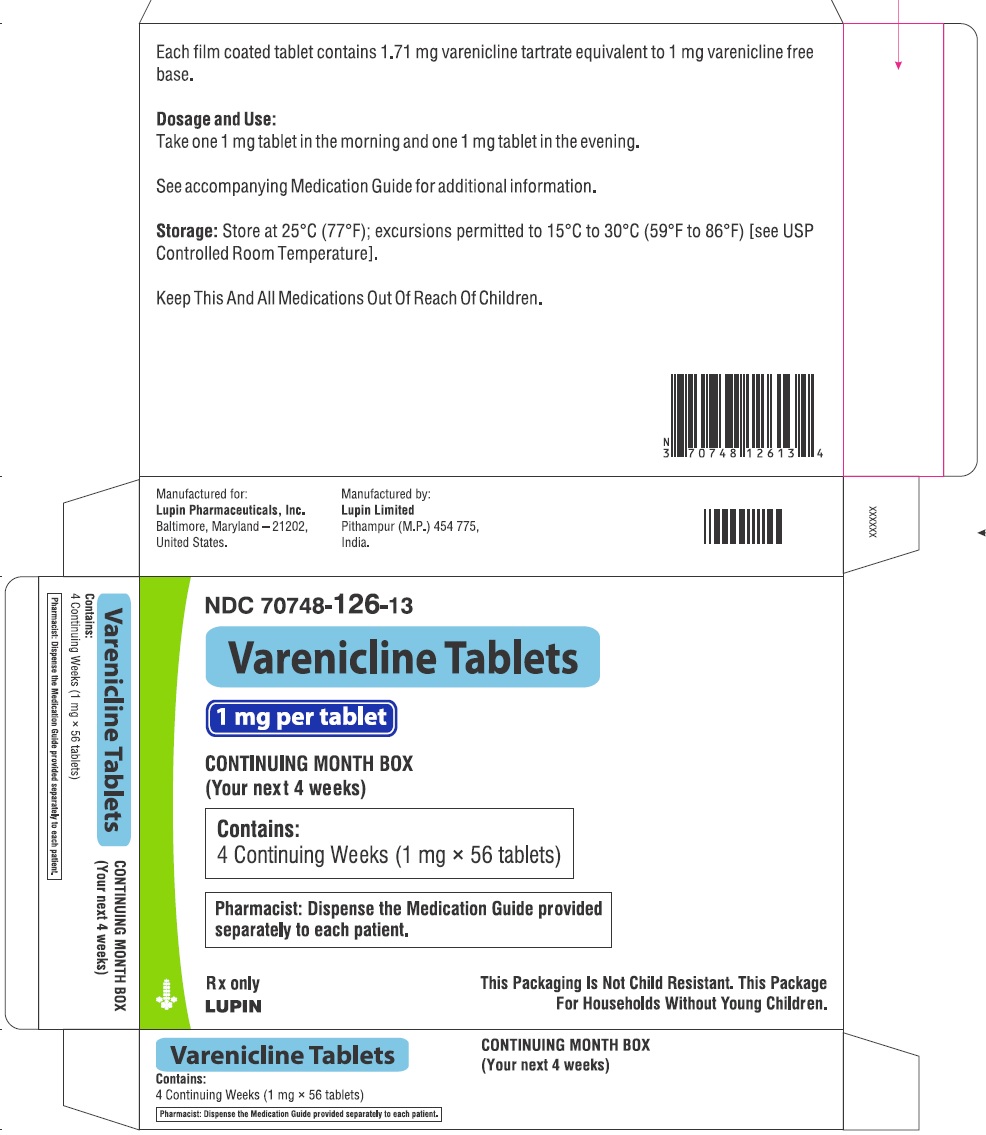
- NDC Code(s): 70748-125-13, 70748-126-13, 70748-127-49, 70748-128-49
- Packager: Lupin Pharmaceuticals, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated March 27, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use VARENICLINE TABLETS safely and effectively. See full prescribing information for VARENICLINE TABLETS.
VARENICLINE Tablets, for oral use
Initial U.S. Approval: 2006INDICATIONS AND USAGE
DOSAGE AND ADMINISTRATION
- Begin varenicline tablets dosing one week before the date set by the patient to stop smoking. Alternatively, the patient can begin varenicline tablets dosing and then quit smoking between days 8 and 35 of treatment. (2.1)
- Starting Week: 0.5 mg once daily on days 1 to 3 and 0.5 mg twice daily on days 4 to 7. (2.1)
- Continuing Weeks : 1 mg twice daily for a total of 12 weeks. (2.1)
- An additional 12 weeks of treatment is recommended for successful quitters to increase likelihood of long-term abstinence. (2.1)
- Consider a gradual approach to quitting smoking with varenicline tablets for patients who are sure that they are not able or willing to quit abruptly. Patients should begin varenicline tablets dosing and reduce smoking by 50% from baseline within the first four weeks, by an additional 50% in the next four weeks, and continue reducing with the goal of reaching complete abstinence by 12 weeks. Continue treatment for an additional 12 weeks, for a total of 24 weeks. (2.1)
- Severe Renal Impairment (estimated creatinine clearance less than 30 mL/min) : Begin with 0.5 mg once daily and titrate to 0.5 mg twice daily. For patients with end-stage renal disease undergoing hemodialysis, a maximum of 0.5 mg daily may be given if tolerated. (2.2)
- Consider dose reduction for patients who cannot tolerate adverse effects. (2.1)
- Another attempt at treatment is recommended for those who fail to stop smoking or relapse when factors contributing to the failed attempt have been addressed. (2.1)
- Provide patients with appropriate educational materials and counseling to support the quit attempt. (2.1)
DOSAGE FORMS AND STRENGTHS
Tablets: 0.5 mg and 1 mg (3)
CONTRAINDICATIONS
History of serious hypersensitivity or skin reactions to varenicline tablets. (4)
WARNINGS AND PRECAUTIONS
- Neuropsychiatric Adverse Events:Postmarketing reports of serious or clinically significant neuropsychiatric adverse events have included changes in mood (including depression and mania), psychosis, hallucinations, paranoia, delusions, homicidal ideation, aggression, hostility, agitation, anxiety, and panic, as well as suicidal ideation, suicide attempt, and completed suicide. Observe patients attempting to quit smoking with varenicline tablets for the occurrence of such symptoms and instruct them to discontinue varenicline tablets and contact a healthcare provider if they experience such adverse events. (5.1)
- Seizures:New or worsening seizures have been observed in patients taking varenicline tablets. Varenicline tablets should be used cautiously in patients with a history of seizures or other factors that can lower the seizure threshold. (5.2)
- Interaction with Alcohol:Increased effects of alcohol have been reported. Instruct patients to reduce the amount of alcohol they consume until they know whether varenicline tablets affect them. (5.3)
- Accidental Injury:Accidental injuries (e.g., traffic accidents) have been reported. Instruct patients to use caution driving or operating machinery until they know how varenicline tablets may affect them. (5.4)
- Cardiovascular Events:Patients with underlying cardiovascular (CV) disease may be at increased risk of CV events; however, these concerns must be balanced with the health benefits of smoking cessation. Instruct patients to notify their healthcare providers of new or worsening CV symptoms and to seek immediate medical attention if they experience signs and symptoms of myocardial infarction (MI) or stroke. (5.5 and 6.1)
- Somnambulism:Cases of somnambulism have been reported in patients taking varenicline tablets. Some cases described harmful behavior to self, others, or property. Instruct patients to discontinue varenicline tablets and notify their healthcare provider if they experience somnambulism. (5.6 and 6.2)
- Angioedema and Hypersensitivity Reactions:Such reactions, including angioedema, infrequently life-threatening, have been reported. Instruct patients to discontinue varenicline tablets and immediately seek medical care if symptoms occur. (5.7 and 6.2)
- Serious Skin Reactions:Rare, potentially life-threatening skin reactions have been reported. Instruct patients to discontinue varenicline tablets and contact a healthcare provider immediately at first appearance of skin rash with mucosal lesions. (5.8 and 6.2)
- Nausea:Nausea is the most common adverse reaction (up to 30% incidence rate). Dose reduction may be helpful. (5.9)
ADVERSE REACTIONS
Most common adverse reactions (>5% and twice the rate seen in placebo-treated patients) were nausea, abnormal (e.g., vivid, unusual, or strange) dreams, constipation, flatulence, and vomiting. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Lupin Pharmaceuticals, Inc. at 1-800-399-2561 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- Other Smoking Cessation Therapies: Safety and efficacy in combination with other smoking cessation therapies has not been established. Coadministration of varenicline and transdermal nicotine resulted in a high rate of discontinuation due to adverse events. (7.1)
- Effect of Smoking Cessation on Other Drugs: Pharmacokinetics or pharmacodynamics of certain drugs (e.g., theophylline, warfarin, insulin) may be altered, necessitating dose adjustment. (7.2)
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 1/2024
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Usual Dosage for Adults
2.2 Dosage in Special Populations
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Neuropsychiatric Adverse Events including Suicidality
5.2 Seizures
5.3 Interaction with Alcohol
5.4 Accidental Injury
5.5 Cardiovascular Events
5.6 Somnambulism
5.7 Angioedema and Hypersensitivity Reactions
5.8 Serious Skin Reactions
5.9 Nausea
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Use with Other Drugs for Smoking Cessation
7.2 Effect of Smoking Cessation on Other Drugs
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
9 DRUG ABUSE AND DEPENDENCE
9.1 Controlled Substance
9.3 Dependence
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Initiation of Abstinence
14.2 Urge to Smoke
14.3 Long-Term Abstinence
14.4 Alternative Instructions for Setting a Quit Date
14.5 Gradual Approach to Quitting Smoking
14.6 Re-Treatment Study
14.7 Subjects with Chronic Obstructive Pulmonary Disease
14.8 Subjects with Cardiovascular Disease and Other Cardiovascular Analyses
14.9 Subjects with Major Depressive Disorder
14.10 Postmarketing Neuropsychiatric Safety Outcome Trial
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Usual Dosage for Adults
Smoking cessation therapies are more likely to succeed for patients who are motivated to stop smoking and who are provided additional advice and support. Provide patients with appropriate educational materials and counseling to support the quit attempt.
The patient should set a date to stop smoking. Begin varenicline tablets dosing one week before this date. Alternatively, the patient can begin varenicline tablets dosing and then quit smoking between days 8 and 35 of treatment.
Varenicline tablets should be taken orally after eating and with a full glass of water.
The recommended dose of varenicline tablets is 1 mg twice daily following a 1-week titration as follows:
Days 1 to 3:
0.5 mg once daily
Days 4 to 7:
0.5 mg twice daily
Days 8 to end of treatment:
1 mg twice daily
Patients should be treated with varenicline tablets for 12 weeks. For patients who have successfully stopped smoking at the end of 12 weeks, an additional course of 12 weeks treatment with varenicline tablets is recommended to further increase the likelihood of long-term abstinence.
For patients who are sure that they are not able or willing to quit abruptly, consider a gradual approach to quitting smoking with varenicline tablets. Patients should begin varenicline tablets dosing and reduce smoking by 50% from baseline within the first four weeks, by an additional 50% in the next four weeks, and continue reducing with the goal of reaching complete abstinence by 12 weeks. Continue varenicline tablets treatment for an additional 12 weeks, for a total of 24 weeks of treatment. Encourage patients to attempt quitting sooner if they feel ready [see Clinical Studies (14.5)].
Patients who are motivated to quit, and who did not succeed in stopping smoking during prior varenicline tablets therapy for reasons other than intolerability due to adverse events or who relapsed after treatment, should be encouraged to make another attempt with varenicline tablets once factors contributing to the failed attempt have been identified and addressed.
Consider a temporary or permanent dose reduction in patients who cannot tolerate the adverse effects of varenicline tablets.
2.2 Dosage in Special Populations
Patients with Impaired Renal Function
No dosage adjustment is necessary for patients with mild to moderate renal impairment. For patients with severe renal impairment (estimated creatinine clearance less than 30 mL per min), the recommended starting dose of varenicline tablets is 0.5 mg once daily. The dose may then be titrated as needed to a maximum dose of 0.5 mg twice daily. For patients with end-stage renal disease undergoing hemodialysis, a maximum dose of 0.5 mg once daily may be administered if tolerated [see Use in Specific Populations (8.6), Clinical Pharmacology (12.3)].
Elderly and Patients with Impaired Hepatic Function
No dosage adjustment is necessary for patients with hepatic impairment. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function [see Use in Specific Populations (8.5)].
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Neuropsychiatric Adverse Events including Suicidality
Serious neuropsychiatric adverse events have been reported in patients being treated with varenicline tablets [see Adverse Reactions (6.2)]. These postmarketing reports have included changes in mood (including depression and mania), psychosis, hallucinations, paranoia, delusions, homicidal ideation, aggression, hostility, agitation, anxiety, and panic, as well as suicidal ideation, suicide attempt, and completed suicide. Some patients who stopped smoking may have been experiencing symptoms of nicotine withdrawal, including depressed mood. Depression, rarely including suicidal ideation, has been reported in smokers undergoing a smoking cessation attempt without medication. However, some of these adverse events occurred in patients taking varenicline tablets who continued to smoke.
Neuropsychiatric adverse events occurred in patients without and with pre-existing psychiatric disease; some patients experienced worsening of their psychiatric illnesses. Some neuropsychiatric adverse events, including unusual and sometimes aggressive behavior directed to oneself or others, may have been worsened by concomitant use of alcohol [see Warnings and Precautions (5.3), Adverse Reactions (6.2)]. Observe patients for the occurrence of neuropsychiatric adverse events. Advise patients and caregivers that the patient should stop taking varenicline tablets and contact a healthcare provider immediately if agitation, depressed mood, or changes in behavior or thinking that are not typical for the patient are observed, or if the patient develops suicidal ideation or suicidal behavior. The healthcare provider should evaluate the severity of the symptoms and the extent to which the patient is benefiting from treatment, and consider options including dose reduction, continued treatment under closer monitoring, or discontinuing treatment. In many postmarketing cases, resolution of symptoms after discontinuation of varenicline tablets were reported. However, the symptoms persisted in some cases; therefore, ongoing monitoring and supportive care should be provided until symptoms resolve.
The neuropsychiatric safety of varenicline tablets was evaluated in a randomized, double-blind, active and placebo-controlled study that included patients without a history of psychiatric disorder (non-psychiatric cohort, N=3912) and patients with a history of psychiatric disorder (psychiatric cohort, N=4003). In the non-psychiatric cohort, varenicline tablets were not associated with an increased incidence of clinically significant neuropsychiatric adverse events in a composite endpoint comprising anxiety, depression, feeling abnormal, hostility, agitation, aggression, delusions, hallucinations, homicidal ideation, mania, panic, and irritability. In the psychiatric cohort, there were more events reported in each treatment group compared to the non-psychiatric cohort, and the incidence of events in the composite endpoint was higher for each of the active treatments compared to placebo: Risk Differences (RDs) (95%CI) vs. placebo were 2.7% (-0.05, 5.4) for varenicline tablets, 2.2% (-0.5, 4.9) for bupropion, and 0.4% (-2.2, 3.0) for transdermal nicotine. In the non-psychiatric cohort, neuropsychiatric adverse events of a serious nature were reported in 0.1% of varenicline tablets -treated patients and 0.4% of placebo-treated patients. In the psychiatric cohort, neuropsychiatric events of a serious nature were reported in 0.6% of varenicline tablets -treated patients, with 0.5% involving psychiatric hospitalization. In placebo-treated patients, serious neuropsychiatric events occurred in 0.6%, with 0.2% requiring psychiatric hospitalization [see Clinical Studies (14.10)].
5.2 Seizures
During clinical trials and the postmarketing experience, there have been reports of seizures in patients treated with varenicline tablets. Some patients had no history of seizures, whereas others had a history of seizure disorder that was remote or well-controlled. In most cases, the seizure occurred within the first month of therapy. Weigh this potential risk against the potential benefits before prescribing varenicline tablets in patients with a history of seizures or other factors that can lower the seizure threshold. Advise patients to discontinue varenicline tablets and contact a healthcare provider immediately if they experience a seizure while on treatment [see Adverse Reactions (6.2)].
5.3 Interaction with Alcohol
There have been postmarketing reports of patients experiencing increased intoxicating effects of alcohol while taking varenicline tablets. Some cases described unusual and sometimes aggressive behavior, and were often accompanied by amnesia for the events. Advise patients to reduce the amount of alcohol they consume while taking varenicline tablets until they know whether varenicline tablets affect their tolerance for alcohol [see Adverse Reactions (6.2)].
5.4 Accidental Injury
There have been postmarketing reports of traffic accidents, near-miss incidents in traffic, or other accidental injuries in patients taking varenicline tablets. In some cases, the patients reported somnolence, dizziness, loss of consciousness or difficulty concentrating that resulted in impairment, or concern about potential impairment, in driving or operating machinery. Advise patients to use caution driving or operating machinery or engaging in other potentially hazardous activities until they know how varenicline tablets may affect them.
5.5 Cardiovascular Events
A comprehensive evaluation of cardiovascular (CV) risk with varenicline tablets suggests that patients with underlying CV disease may be at increased risk; however, these concerns must be balanced with the health benefits of smoking cessation. CV risk has been assessed for varenicline tablets in randomized controlled trials (RCT) and meta-analyses of RCTs. In a smoking cessation trial in patients with stable CV disease, CV events were infrequent overall; however, nonfatal myocardial infarction (MI) and nonfatal stroke occurred more frequently in patients treated with varenicline tablets compared to placebo. All-cause and CV mortality was lower in patients treated with varenicline tablets [see Clinical Studies (14.8)]. This study was included in a meta-analysis of 15 varenicline tablets efficacy trials in various clinical populations that showed an increased hazard ratio for Major Adverse Cardiovascular Events (MACE) of 1.95; however, the finding was not statistically significant (95% CI: 0.79, 4.82). In the large postmarketing neuropsychiatric safety outcome trial, an analysis of adjudicated MACE events was conducted for patients while in the trial and during a 28-week non-treatment extension period. Few MACE events occurred during the trial; therefore, the findings did not contribute substantively to the understanding of CV risk with varenicline tablets. Instruct patients to notify their healthcare providers of new or worsening CV symptoms and to seek immediate medical attention if they experience signs and symptoms of MI or stroke [see Clinical Studies (14.10)].
5.6 Somnambulism
Cases of somnambulism have been reported in patients taking varenicline tablets. Some cases described harmful behavior to self, others, or property. Instruct patients to discontinue varenicline tablets and notify their healthcare provider if they experience somnambulism [see Adverse Reactions (6.2)].
5.7 Angioedema and Hypersensitivity Reactions
There have been postmarketing reports of hypersensitivity reactions including angioedema in patients treated with varenicline tablets [see Adverse Reactions (6.2), Patient Counseling Information (17)]. Clinical signs included swelling of the face, mouth (tongue, lips, and gums), extremities, and neck (throat and larynx). There were infrequent reports of life-threatening angioedema requiring emergent medical attention due to respiratory compromise. Instruct patients to discontinue varenicline tablets and immediately seek medical care if they experience these symptoms.
5.8 Serious Skin Reactions
There have been postmarketing reports of rare but serious skin reactions, including Stevens-Johnson Syndrome and erythema multiforme, in patients using varenicline tablets [see Adverse Reactions (6.2)]. As these skin reactions can be life-threatening, instruct patients to stop taking varenicline tablets and contact a healthcare provider immediately at the first appearance of a skin rash with mucosal lesions or any other signs of hypersensitivity.
5.9 Nausea
Nausea was the most common adverse reaction reported with varenicline tablets treatment. Nausea was generally described as mild or moderate and often transient; however, for some patients, it was persistent over several months. The incidence of nausea was dose-dependent. Initial dose-titration was beneficial in reducing the occurrence of nausea. For patients treated to the maximum recommended dose of 1 mg twice daily following initial dosage titration, the incidence of nausea was 30% compared with 10% in patients taking a comparable placebo regimen. In patients taking varenicline tablets 0.5 mg twice daily following initial titration, the incidence was 16% compared with 11% for placebo. Approximately 3% of patients treated with varenicline tablets 1 mg twice daily in studies involving 12 weeks of treatment discontinued treatment prematurely because of nausea. For patients with intolerable nausea, a dose reduction should be considered.
-
6 ADVERSE REACTIONS
The following serious adverse reactions were reported in postmarketing experience and are discussed in greater detail in other sections of the labeling:
- Neuropsychiatric Adverse Events including Suicidality [see Warnings and Precautions (5.1)]
- Seizures[see Warnings and Precautions (5.2)]
- Interaction with Alcohol [see Warnings and Precautions (5.3)]
- Accidental Injury [see Warnings and Precautions (5.4)]
- Cardiovascular Events [see Warnings and Precautions (5.5)]
- Somnambulism [see Warnings and Precautions (5.6)]
- Angioedema and Hypersensitivity Reactions [see Warnings and Precautions (5.7)]
- Serious Skin Reactions [see Warnings and Precautions (5.8)]
In the placebo-controlled premarketing studies, the most common adverse events associated with varenicline tablets (>5% and twice the rate seen in placebo-treated patients) were nausea, abnormal (vivid, unusual, or strange) dreams, constipation, flatulence, and vomiting.
The treatment discontinuation rate due to adverse events in patients dosed with 1 mg twice daily was 12% for varenicline tablets, compared to 10% for placebo in studies of three months' treatment. In this group, the discontinuation rates that are higher than placebo for the most common adverse events in varenicline tablets -treated patients were as follows: nausea (3% vs. 0.5% for placebo), insomnia (1.2% vs. 1.1% for placebo), and abnormal dreams (0.3% vs. 0.2% for placebo).
Smoking cessation, with or without treatment, is associated with nicotine withdrawal symptoms and has also been associated with the exacerbation of underlying psychiatric illness.
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, the adverse reactions rates observed in the clinical studies of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
During the premarketing development of varenicline tablets, over 4500 subjects were exposed to varenicline tablets, with over 450 treated for at least 24 weeks and approximately 100 for a year. Most study participants were treated for 12 weeks or less.
The most common adverse event associated with varenicline tablets treatment is nausea, occurring in 30% of patients treated at the recommended dose, compared with 10% in patients taking a comparable placebo regimen [see Warnings and Precautions (5.9)].
Table 1 shows the adverse events for varenicline tablets and placebo in the 12- week fixed dose premarketing studies with titration in the first week [Studies 2 (titrated arm only), 4, and 5]. Adverse events were categorized using the Medical Dictionary for Regulatory Activities (MedDRA, Version 7.1).
MedDRA High Level Group Terms (HLGT) reported in ≥ 5% of patients in the varenicline tablets 1 mg twice daily dose group, and more commonly than in the placebo group, are listed, along with subordinate Preferred Terms (PT) reported in ≥ 1% of varenicline tablets patients (and at least 0.5% more frequent than placebo). Closely related Preferred Terms such as 'Insomnia', 'Initial insomnia', 'Middle insomnia', 'Early morning awakening' were grouped, but individual patients reporting two or more grouped events are only counted once.
Table 1: Common Treatment Emergent AEs (%) in the Fixed-Dose, Placebo-Controlled Studies (HLGTs >5% of Patients in the 1 mg BID Varenicline Tablets Group and More Commonly than Placebo and PT ≥ 1% in the 1 mg BID Varenicline Tablets Group, and 1 mg BID Varenicline Tablets at Least 0.5% More than Placebo) * Includes PTs Abdominal (pain, pain upper, pain lower, discomfort, tenderness, distension) and Stomach discomfort
** Includes PTs Insomnia/Initial insomnia/Middle insomnia/Early morning awakening
SYSTEM ORGAN CLASS
High Level Group Term
Preferred Term
Varenicline Tablets
0.5 mg BID
N=129
Varenicline Tablets
1 mg BID
N=821
Placebo
N=805
GASTROINTESTINAL (GI)
GI Signs and Symptoms
Nausea
16
30
10
Abdominal Pain *
5
7
5
Flatulence
9
6
3
Dyspepsia
5
5
3
Vomiting
1
5
2
GI Motility/Defecation
Conditions
Constipation
5
8
3
Gastroesophageal reflux
disease
1
1
0
Salivary Gland Conditions
Dry mouth
4
6
4
PSYCHIATRIC DISORDERS
Sleep
Disorder/Disturbances
Insomnia **
19
18
13
Abnormal dreams
9
13
5
Sleep disorder
2
5
3
Nightmare
2
1
0
NERVOUS SYSTEM
Headaches
Headache
19
15
13
Neurological Disorders
NEC
Dysgeusia
8
5
4
Somnolence
3
3
2
Lethargy
2
1
0
GENERAL DISORDERS
General Disorders NEC
Fatigue/Malaise/Asthenia
4
7
6
RESPIR/THORACIC/MEDIAST
Respiratory Disorders NEC
Rhinorrhea
0
1
0
Dyspnea
2
1
1
Upper Respiratory Tract
Disorder
7
5
4
SKIN/SUBCUTANEOUS TISSUE
Epidermal and Dermal
Conditions
Rash
1
3
2
Pruritis
0
1
1
METABOLISM & NUTRITION
Appetite/General Nutrition
Disorders
Increased appetite
4
3
2
Decreased appetite/
Anorexia
1
2
1
The overall pattern and frequency of adverse events during the longer-term premarketing trials was similar to those described in Table 1, though several of the most common events were reported by a greater proportion of patients with long-term use (e.g., nausea was reported in 40% of patients treated with varenicline tablets 1 mg twice daily in a one year study, compared to 8% of placebo-treated patients).
Following is a list of treatment-emergent adverse events reported by patients treated with varenicline tablets during all premarketing clinical trials and updated based on pooled data from 18 placebo-controlled pre- and postmarketing studies, including approximately 5,000 patients treated with varenicline. Adverse events were categorized using MedDRA, Version 16.0. The listing does not include those events already listed in the previous tables or elsewhere in labeling, those events for which a drug cause was remote, those events which were so general as to be uninformative, and those events reported only once which did not have a substantial probability of being acutely life-threatening.
Blood and Lymphatic System Disorders. Infrequent: anemia, lymphadenopathy. Rare: leukocytosis, splenomegaly, thrombocytopenia.
Cardiac Disorders. Infrequent: angina pectoris, myocardial infarction, palpitations, tachycardia. Rare: acute coronary syndrome, arrhythmia, atrial fibrillation, bradycardia, cardiac flutter, cor pulmonale, coronary artery disease, ventricular extrasystoles.
Ear and Labyrinth Disorders. Infrequent: tinnitus, vertigo. Rare: deafness, Meniere's disease.
Endocrine Disorders. Infrequent: thyroid gland disorders.
Eye Disorders. Infrequent: conjunctivitis, eye irritation, eye pain, vision blurred, visual impairment. Rare: blindness transient, cataract subcapsular, dry eye, night blindness, ocular vascular disorder, photophobia, vitreous floaters.
Gastrointestinal Disorders. Frequent: diarrhea, toothache. Infrequent: dysphagia, eructation, gastritis, gastrointestinal hemorrhage, mouth ulceration. Rare: enterocolitis, esophagitis, gastric ulcer, intestinal obstruction, pancreatitis acute.
General Disorders and Administration Site Conditions. Frequent: chest pain. Infrequent: chest discomfort, chills, edema, influenza-like illness, pyrexia.
Hepatobiliary Disorders. Rare: gall bladder disorder.
Investigations. Frequent: liver function test abnormal, weight increased. Infrequent: electrocardiogram abnormal. Rare: muscle enzyme increased, urine analysis abnormal.
Metabolism and Nutrition Disorders. Infrequent: diabetes mellitus, hypoglycemia. Rare: hyperlipidemia, hypokalemia.
Musculoskeletal and Connective Tissue Disorders. Frequent: arthralgia, back pain, myalgia. Infrequent: arthritis, muscle cramp, musculoskeletal pain. Rare: myositis, osteoporosis.
Nervous System Disorders. Frequent: disturbance in attention, dizziness. Infrequent: amnesia, convulsion, migraine, parosmia, syncope, tremor. Rare: balance disorder, cerebrovascular accident, dysarthria, mental impairment, multiple sclerosis, VIIth nerve paralysis, nystagmus, psychomotor hyperactivity, psychomotor skills impaired, restless legs syndrome, sensory disturbance, transient ischemic attack, visual field defect.
Psychiatric Disorders. Infrequent: dissociation, libido decreased, mood swings, thinking abnormal. Rare: bradyphrenia, disorientation, euphoric mood.
Renal and Urinary Disorders. Infrequent: nocturia, pollakiuria, urine abnormality. Rare: nephrolithiasis, polyuria, renal failure acute, urethral syndrome, urinary retention.
Reproductive System and Breast Disorders. Frequent: menstrual disorder. Infrequent: erectile dysfunction. Rare: sexual dysfunction.
Respiratory, Thoracic and Mediastinal Disorders. Frequent: respiratory disorders. Infrequent: asthma, epistaxis, rhinitis allergic, upper respiratory tract inflammation. Rare: pleurisy, pulmonary embolism.
Skin and Subcutaneous Tissue Disorders. Infrequent: acne, dry skin, eczema, erythema, hyperhidrosis, urticaria. Rare: photosensitivity reaction, psoriasis.
Vascular Disorders. Infrequent: hot flush. Rare: thrombosis.
Varenicline tablets have also been studied in postmarketing trials including (1) a trial conducted in patients with chronic obstructive pulmonary disease (COPD), (2) a trial conducted in generally healthy patients (similar to those in the premarketing studies) in which they were allowed to select a quit date between days 8 and 35 of treatment ("alternative quit date instruction trial"), (3) a trial conducted in patients who did not succeed in stopping smoking during prior varenicline tablets therapy, or who relapsed after treatment ("re-treatment trial"), (4) a trial conducted in patients with stable cardiovascular disease, (5) a trial conducted in patients with stable schizophrenia or schizoaffective disorder, (6) a trial conducted in patients with major depressive disorder, (7) a postmarketing neuropsychiatric safety outcome trial in patients without or with a history of psychiatric disorder, (8) a non-treatment extension of the postmarketing neuropsychiatric safety outcome trial that assessed CV safety, (9) a trial in patients who were not able or willing to quit abruptly and who were instructed to quit gradually ("gradual approach to quitting smoking trial").
Adverse events in the trial of patients with COPD (1), in the alternative quit date instruction trial (2), and in the gradual approach to quitting smoking trial (9) were similar to those observed in premarketing studies. In the re-treatment trial (3), the profile of common adverse events was similar to that previously reported, but, in addition, varenicline -treated patients also commonly reported diarrhea (6% vs. 4% in placebo-treated patients), depressed mood disorders and disturbances (6% vs. 1%), and other mood disorders and disturbances (5% vs. 2%).
In the trial of patients with stable cardiovascular disease (4), more types and a greater number of cardiovascular events were reported compared to premarketing studies, as shown in Table 1 and in Table 2 below.
Table 2: Cardiovascular Mortality and Nonfatal Cardiovascular Events (%) with a Frequency >1% in Either Treatment Group in the Trial of Patients with Stable Cardiovascular Disease *some procedures were part of management of nonfatal MI and hospitalization for angina
Varenicline Tablets
1 mg BID
N=353
Placebo
N=350
Adverse Events ≥1% in either treatment group
Up to 30 days after treatment
Angina pectoris
3.7
2.0
Chest pain
2.5
2.3
Peripheral edema
2.0
1.1
Hypertension
1.4
2.6
Palpitations
0.6
1.1
Adjudicated Cardiovascular Mortality (up to 52 weeks)
0.3
0.6
Adjudicated Nonfatal Serious Cardiovascular Events ≥1% in either treatment group
Up to 30 days after treatment
Nonfatal MI
1.1
0.3
Hospitalization for angina pectoris
0.6
1.1
Beyond 30 days after treatment and up to 52 weeks
Need for coronary revascularization*
2.0
0.6
Hospitalization for angina pectoris
1.7
1.1
New diagnosis of peripheral vascular disease (PVD)
or admission for a PVD procedure
1.4
0.6
In the trial of patients with stable schizophrenia or schizoaffective disorder (5), 128 smokers on antipsychotic medication were randomized 2:1 to varenicline (1 mg twice daily) or placebo for 12 weeks with 12-week non-drug follow-up. The most common treatment emergent adverse events reported in this trial are shown in Table 3 below.
Table 3: Common Treatment Emergent AEs (%) in the Trial of Patients with Stable Schizophrenia or Schizoaffective Disorder Varenicline Tablets
1 mg BID
N=84
Placebo
N=43
Adverse Events ≥10% in the varenicline group
Nausea
24
14
Headache
11
19
Vomiting
11
9
Psychiatric Adverse Events ≥5% and at a higher rate than in the placebo group
Insomnia
10
5
For the trial of patients with major depressive disorder (6), the most common treatment emergent adverse events reported are shown in Table 4 below. Additionally, in this trial, patients treated with varenicline were more likely than patients treated with placebo to report one of events related to hostility and aggression (3% vs. 1%).
Table 4: Common Treatment Emergent AEs (%) in the Trial of Patients with Major Depressive Disorder Varenicline Tablets
1 mg BID
N=256
Placebo
N=269
Adverse Events ≥10% in either treatment group
Nausea
27
10
Headache
17
11
Abnormal dreams
11
8
Insomnia
11
5
Irritability
11
8
Psychiatric Adverse Events ≥2% in any treatment group and not included above
Depressed mood disorders and disturbances
11
9
Anxiety
7
9
Agitation
7
4
Tension
4
3
Hostility
2
0.4
Restlessness
2
2
In the trial of patients without or with a history of psychiatric disorder (7), the most common adverse events in subjects treated with varenicline were similar to those observed in premarketing studies. Most common treatment-emergent adverse events reported in this trial are shown in Table 5 below.
Table 5: Treatment Emergent Common AEs (%) in the Trial of Patients without or with a History of Psychiatric Disorder Varenicline Tablets
1 mg BID
Placebo
Adverse Events ≥10% in the varenicline group
Entire study population, N
1982
1979
Nausea
25
7
Headache
12
10
Psychiatric Adverse Events ≥2% in any treatment group
Non-psychiatric cohort, N
975
982
Abnormal dreams
8
4
Agitation
3
3
Anxiety
5
6
Depressed mood
3
3
Insomnia
10
7
Irritability
3
4
Sleep disorder
3
2
Psychiatric cohort, N
1007
997
Abnormal dreams
12
5
Agitation
5
4
Anxiety
8
6
Depressed mood
5
5
Depression
5
5
Insomnia
9
7
Irritability
5
7
Nervousness
2
3
Sleep disorder
3
2
In the non-treatment extension of the postmarketing neuropsychiatric safety outcomes trial that assessed CV safety (8), the most common adverse events in subjects treated with varenicline and occurring up to 30 days after last dose of treatment were similar to those observed in premarketing studies.
6.2 Postmarketing Experience
The following adverse events have been reported during post-approval use of varenicline tablets. Because these events are reported voluntarily from a population of uncertain size, it is not possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
There have been reports of depression, mania, psychosis, hallucinations, paranoia, delusions, homicidal ideation, aggression, hostility, anxiety, and panic, as well as suicidal ideation, suicide attempt, and completed suicide in patients attempting to quit smoking while taking varenicline tablets [see Warnings and Precautions (5.1)].
There have been postmarketing reports of new or worsening seizures in patients treated with varenicline tablets [see Warnings and Precautions (5.2)].
There have been postmarketing reports of patients experiencing increased intoxicating effects of alcohol while taking varenicline tablets. Some reported neuropsychiatric events, including unusual and sometimes aggressive behavior [see Warnings and Precautions (5.1) and (5.3)].
There have been reports of hypersensitivity reactions, including angioedema [see Warnings and Precautions (5.7)].
There have also been reports of serious skin reactions, including Stevens-Johnson Syndrome and erythema multiforme, in patients taking varenicline tablets [see Warnings and Precautions (5.8)].
There have been reports of myocardial infarction (MI) and cerebrovascular accident (CVA) including ischemic and hemorrhagic events in patients taking varenicline tablets. In the majority of the reported cases, patients had pre-existing cardiovascular disease and/or other risk factors. Although smoking is a risk factor for MI and CVA, based on temporal relationship between medication use and events, a contributory role of varenicline cannot be ruled out [see Warnings and Precautions (5.5)].
There have been reports of hyperglycemia in patients following initiation of varenicline tablets.
There have been reports of somnambulism, some resulting in harmful behavior to self, others, or property in patients treated with varenicline tablets [see Warnings and Precautions (5.6)].
-
7 DRUG INTERACTIONS
Based on varenicline characteristics and clinical experience to date, varenicline tablets has no clinically meaningful pharmacokinetic drug interactions [see Clinical Pharmacology (12.3)].
7.1 Use with Other Drugs for Smoking Cessation
Safety and efficacy of varenicline tablets in combination with other smoking cessation therapies have not been studied.
Bupropion
Varenicline (1 mg twice daily) did not alter the steady-state pharmacokinetics of bupropion (150 mg twice daily) in 46 smokers. The safety of the combination of bupropion and varenicline has not been established.
Nicotine replacement therapy (NRT)
Although co-administration of varenicline (1 mg twice daily) and transdermal nicotine (21 mg/day) for up to 12 days did not affect nicotine pharmacokinetics, the incidence of nausea, headache, vomiting, dizziness, dyspepsia, and fatigue was greater for the combination than for NRT alone. In this study, eight of twenty-two (36%) patients treated with the combination of varenicline and NRT prematurely discontinued treatment due to adverse events, compared to 1 of 17 (6%) of patients treated with NRT and placebo.
7.2 Effect of Smoking Cessation on Other Drugs
Physiological changes resulting from smoking cessation, with or without treatment with varenicline tablets, may alter the pharmacokinetics or pharmacodynamics of certain drugs (e.g., theophylline, warfarin, insulin) for which dosage adjustment may be necessary.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Available data have not suggested an increased risk for major birth defects following exposure to varenicline in pregnancy, compared with women who smoke [see Data]. Smoking during pregnancy is associated with maternal, fetal, and neonatal risks (see Clinical Considerations). In animal studies, varenicline did not result in major malformations but caused decreased fetal weights in rabbits when dosed during organogenesis at exposures equivalent to 50 times the exposure at the maximum recommended human dose (MRHD). Additionally, administration of varenicline to pregnant rats during organogenesis through lactation produced developmental toxicity in offspring at maternal exposures equivalent to 36 times human exposure at the MRHD [see Data].
The estimated background risk of oral clefts is increased by approximately 30% in infants of women who smoke during pregnancy, compared to pregnant women who do not smoke. The background risk of other major birth defects and miscarriage for the indicated population are unknown. In the US general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Clinical Considerations
Disease-Associated Maternal and/or Embryo/Fetal Risk
Smoking during pregnancy causes increased risks of orofacial clefts, premature rupture of membranes, placenta previa, placental abruption, ectopic pregnancy, fetal growth restriction and low birth weight, stillbirth, preterm delivery and shortened gestation, neonatal death, sudden infant death syndrome and reduction of lung function in infants. It is not known whether quitting smoking with varenicline tablets during pregnancy reduces these risks.
Data
Human Data
A population-based observational cohort study using the national registers of Denmark and Sweden compared pregnancy and birth outcomes among women exposed to varenicline (N=335, includes 317 first trimester exposed) with women who smoked during pregnancy (N=78,412) and with non-smoking pregnant women (N=806,438). The prevalence of major malformations, the primary outcome, was similar in all groups, including between smoking and non-smoking groups. The prevalence of adverse perinatal outcomes in the varenicline-exposed cohort was not greater than in the cohort of women who smoked, and differed somewhat between the three cohorts. The prevalences of the primary and secondary outcomes are shown in Table 6.
Table 6: Summary of Primary and Secondary Outcomes for Three Birth Cohorts *Included only live births in the cohorts. Prevalence among first trimester varenicline-exposed pregnancies (11/317 [3.5%]).
**There was a lag in death data in Denmark, so the cohorts were smaller.
Outcome
Varenicline Cohort
(n=335)
Smoking Cohort
(n=78,412)
Non-Smoking Cohort
(n=806,438)
Major congenital malformation*
12 / 334 (3.6%)
3,382 / 78,028 (4.3%)
33,950 /804,020 (4.2%)
Stillbirth
1 (0.3%)
384 (0.5%)
2,418 (0.3%)
Small for gestational age
42 (12.5%)
13,433 (17.1%)
73,135 (9.1%)
Preterm birth
25 (7.5%)
6,173 (7.9%)
46,732 (5.8%)
Premature rupture of membranes
12 (3.6%)
4,246 (5.4%)
30,641 (3.8%)
Sudden infant death syndrome**
0/307 (0.0%)
51/71,720 (0.1%)
58/755,939 (<0.1%)
The study limitations include the inability to capture malformations in pregnancies that do not result in a live birth, and possible misclassification of outcome and of exposure to varenicline or to smoking.
Other small epidemiological studies of pregnant women exposed to varenicline did not identify an association with major malformations, consistent with the Danish and Swedish observational cohort study. Methodological limitations of these studies include small samples and lack of adequate controls.
Overall, available studies cannot definitely establish or exclude any varenicline-associated risk during pregnancy.
Animal Data
Pregnant rats and rabbits received varenicline succinate during organogenesis at oral doses up to 15 and 30 mg/kg/day, respectively. While no fetal structural abnormalities occurred in either species, maternal toxicity, characterized by reduced body weight gain, and reduced fetal weights occurred in rabbits at the highest dose (exposures 50 times the human exposure at the MRHD of 1 mg twice daily based on AUC). Fetal weight reduction did not occur in rabbits at exposures 23 times the human exposure at the MRHD based on AUC.
In a pre- and postnatal development study, pregnant rats received up to 15 mg/kg/day of oral varenicline succinate from organogenesis through lactation. Maternal toxicity, characterized by a decrease in body weight gain was observed at 15 mg/kg/day (36 times the human exposure at the MRHD based on AUC). However, decreased fertility and increased auditory startle response occurred in offspring at the highest maternal dose of 15 mg/kg/day.
8.2 Lactation
There are no data on the presence of varenicline in human milk, the effects on the breastfed infant, or the effects on milk production. In animal studies varenicline was present in milk of lactating rats [see Data]. However, due to species-specific differences in lactation physiology, animal data may not reliably predict drug levels in human milk. The lack of clinical data during lactation precludes a clear determination of the risk of varenicline tablets to an infant during lactation; however the developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for varenicline tablets and any potential adverse effects on the breastfed child from varenicline tablets or from the underlying maternal condition.
Clinical Considerations
Because there are no data on the presence of varenicline in human milk and the effects on the breastfed infant, breastfeeding women should monitor their infant for seizures and excessive vomiting, which are adverse reactions that have occurred in adults that may be clinically relevant in breastfeeding infants.
Data
In a pre- and postnatal development study, pregnant rats received up to 15 mg/kg/day of oral varenicline succinate through gestation and lactation Mean serum concentrations of varenicline in the nursing pups were 5 to 22% of maternal serum concentrations.
8.4 Pediatric Use
Varenicline is not recommended for use in pediatric patients 16 years of age or younger because its efficacy in this population was not demonstrated.
Single and multiple-dose pharmacokinetics of varenicline have been investigated in pediatric patients aged 12 to 17 years old (inclusive) and were approximately dose proportional over the 0.5 mg to 2 mg daily dose range studied. Steady-state systemic exposure in adolescent patients of bodyweight >55 kg, as assessed by AUC (0 to 24), was comparable to that noted for the same doses in the adult population. When 0.5 mg BID was given, steady-state daily exposure of varenicline was, on average, higher (by approximately 40%) in adolescent patients with bodyweight ≤ 55 kg compared to that noted in the adult population.
The efficacy and safety of varenicline was evaluated in a randomized, double-blind, placebo-controlled study of 312 patients aged 12 to 19 years, who smoked an average of at least 5 cigarettes per day during the 30 days prior to recruitment, had a score of at least 4 on the Fagerstrom Test for Nicotine Dependence scale, and at least one previous failed quit attempt. Patients were stratified by age (12 to 16 years of age, n=216 and 17 to 19 years of age, n=96) and by body weight (≤55 kg and >55 kg). Patients were randomized to one of two doses of varenicline, adjusted by weight to provide plasma levels in the efficacious range (based on adult studies) and placebo. Patients received treatment for 12 weeks, followed by a non-treatment period of 40 weeks, along with age-appropriate counseling throughout the study. Results from this study showed that varenicline, at either dose studied, did not improve continuous abstinence rates at weeks 9 through 12 of treatment compared with placebo in subjects 12 to 19 years of age. The varenicline safety profile in this study was consistent with that observed in adult studies.
8.5 Geriatric Use
A combined single- and multiple-dose pharmacokinetic study demonstrated that the pharmacokinetics of 1 mg varenicline given once daily or twice daily to 16 healthy elderly male and female smokers (aged 65 to 75 years) for 7 consecutive days was similar to that of younger subjects. No overall differences in safety or effectiveness were observed between these subjects and younger subjects, and other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
Varenicline is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function [see Dosage and Administration (2.2)].
No dosage adjustment is recommended for elderly patients.
8.6 Renal Impairment
Varenicline is substantially eliminated by renal glomerular filtration along with active tubular secretion. Dose reduction is not required in patients with mild to moderate renal impairment. For patients with severe renal impairment (estimated creatinine clearance <30 mL/min), and for patients with end-stage renal disease undergoing hemodialysis, dosage adjustment is needed [see Dosage and Administration (2.2), Clinical Pharmacology (12.3)].
-
9 DRUG ABUSE AND DEPENDENCE
9.3 Dependence
Fewer than 1 out of 1,000 patients reported euphoria in clinical trials with varenicline tablets. At higher doses (greater than 2 mg), varenicline tablets produced more frequent reports of gastrointestinal disturbances such as nausea and vomiting. There is no evidence of dose-escalation to maintain therapeutic effects in clinical studies, which suggests that tolerance does not develop. Abrupt discontinuation of varenicline tablets was associated with an increase in irritability and sleep disturbances in up to 3% of patients. This suggests that, in some patients, varenicline may produce mild physical dependence which is not associated with addiction.
In a human laboratory abuse liability study, a single oral dose of 1 mg varenicline did not produce any significant positive or negative subjective responses in smokers. In non-smokers, 1 mg varenicline produced an increase in some positive subjective effects, but this was accompanied by an increase in negative adverse effects, especially nausea. A single oral dose of 3 mg varenicline uniformly produced unpleasant subjective responses in both smokers and non-smokers.
Animals
Studies in rodents have shown that varenicline produces behavioral responses similar to those produced by nicotine. In rats trained to discriminate nicotine from saline, varenicline produced full generalization to the nicotine cue. In self-administration studies, the degree to which varenicline substitutes for nicotine is dependent upon the requirement of the task. Rats trained to self-administer nicotine under easy conditions continued to self-administer varenicline to a degree comparable to that of nicotine; however in a more demanding task, rats self-administered varenicline to a lesser extent than nicotine. Varenicline pretreatment also reduced nicotine self- administration.
-
10 OVERDOSAGE
In case of overdose, standard supportive measures should be instituted as required.
Varenicline has been shown to be dialyzed in patients with end-stage renal disease [see Clinical Pharmacology (12.3)], however, there is no experience in dialysis following overdose.
-
11 DESCRIPTION
Varenicline tablets contain varenicline (as the tartrate salt), which is a partial nicotinic agonist selective for α4β2 nicotinic acetylcholine receptor subtypes.
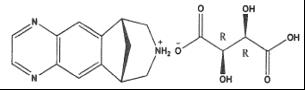
Varenicline, as the tartrate salt, is a powder which is a white to light brown color powder with the following chemical name: 7,8,9,10-tetrahydro-6,10-methano 6H-pyrazino[2,3 h][3]benzazepine, (2R,3R)-2,3-dihydroxybutanedioate (1:1). It is freely soluble in water. Varenicline tartrate has a molecular weight of 361.35 Daltons, and a molecular formula of C13H13N3 • C4H6O6. The chemical structure is:
Varenicline tablets are supplied for oral administration in two strengths: 0.5 mg white to off white, round, biconvex-coated tablets, debossed with "T" on one side and "2" on the other side and 1 mg yellow, round, biconvex-coated tablets, debossed with "T" on one side and "1" on the other side. Each 0.5 mg varenicline tablet contains 0.855 mg of varenicline tartrate equivalent to 0.5 mg of varenicline free base; each 1 mg varenicline tablet contains 1.710 mg of varenicline tartrate equivalent to 1 mg of varenicline free base. The following inactive ingredients are included in the tablets: anhydrous dibasic calcium phosphate, croscarmellose sodium, hypromellose, magnesium stearate, maltodextrin, talc and titanium dioxide. The 1 mg tablet also contains iron oxide yellow.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Varenicline binds with high affinity and selectivity at α4β2 neuronal nicotinic acetylcholine receptors. The efficacy of varenicline tablets in smoking cessation is believed to be the result of varenicline's activity at α4β2 sub-type of the nicotinic receptor where its binding produces agonist activity, while simultaneously preventing nicotine binding to these receptors.
Electrophysiology studies in vitro and neurochemical studies in vivo have shown that varenicline binds to α4β2 neuronal nicotinic acetylcholine receptors and stimulates receptor-mediated activity, but at a significantly lower level than nicotine. Varenicline blocks the ability of nicotine to activate α4β2 receptors and thus to stimulate the central nervous mesolimbic dopamine system, believed to be the neuronal mechanism underlying reinforcement and reward experienced upon smoking. Varenicline is highly selective and binds more potently to α4β2 receptors than to other common nicotinic receptors (>500-fold α3β4, >3,500-fold α7, >20,000-fold α1βγδ), or to non nicotinic receptors and transporters (>2,000-fold). Varenicline also binds with moderate affinity (Ki = 350 nM) to the 5-HT3 receptor.
12.3 Pharmacokinetics
Maximum plasma concentrations of varenicline occur typically within 3 to 4 hours after oral administration. Following administration of multiple oral doses of varenicline, steady-state conditions were reached within 4 days. Over the recommended dosing range, varenicline exhibits linear pharmacokinetics after single or repeated doses.
In a mass balance study, absorption of varenicline was virtually complete after oral administration and systemic availability was ~90%.
Food Effect
Oral bioavailability of varenicline is unaffected by food or time-of-day dosing.
Distribution
Plasma protein binding of varenicline is low (≤20%) and independent of both age and renal function.
Elimination
The elimination half-life of varenicline is approximately 24 hours.
Metabolism
Varenicline undergoes minimal metabolism, with 92% excreted unchanged in the urine.
Excretion
Renal elimination of varenicline is primarily through glomerular filtration along with active tubular secretion possibly via the organic cation transporter, OCT2.
Specific Populations
There are no clinically meaningful differences in varenicline pharmacokinetics due to age, race, gender, smoking status, or use of concomitant medications, as demonstrated in specific pharmacokinetic studies and in population pharmacokinetic analyses.
Age: Geriatric Patients
A combined single- and multiple-dose pharmacokinetic study demonstrated that the pharmacokinetics of 1 mg varenicline given once daily or twice daily to 16 healthy elderly male and female smokers (aged 65 to 75 years) for 7 consecutive days was similar to that of younger subjects.
Age: Pediatric Patients
Varenicline tablets are not recommended for use in pediatric patients 16 years of age or younger because its efficacy in this population was not demonstrated [see Use in Specific Populations (8.4)].
Renal Impairment
Varenicline pharmacokinetics were unchanged in subjects with mild renal impairment (estimated creatinine clearance >50 mL/min and ≤80 mL/min). In subjects with moderate renal impairment (estimated creatinine clearance ≥30 mL/min and ≤50 mL/min), varenicline exposure increased 1.5-fold compared with subjects with normal renal function (estimated creatinine clearance >80 mL/min). In subjects with severe renal impairment (estimated creatinine clearance <30 mL/min), varenicline exposure was increased 2.1-fold. In subjects with end-stage-renal disease (ESRD) undergoing a three-hour session of hemodialysis for three days a week, varenicline exposure was increased 2.7-fold following 0.5 mg once daily administration for 12 days. The plasma Cmax and AUC of varenicline noted in this setting were similar to those of healthy subjects receiving 1 mg twice daily [see Dosage and Administration (2.2), Use in Specific Populations (8.6)]. Additionally, in subjects with ESRD, varenicline was efficiently removed by hemodialysis [see Overdosage (10)].
Hepatic Impairment
Due to the absence of significant hepatic metabolism, varenicline pharmacokinetics should be unaffected in patients with hepatic impairment.
Drug-Drug Interactions
In vitro studies demonstrated that varenicline does not inhibit the following cytochrome P450 enzymes (IC50 >6400 ng/mL): 1A2, 2A6, 2B6, 2C8, 2C9, 2C19, 2D6, 2E1, and 3A4/5. Also, in human hepatocytes in vitro, varenicline does not induce the cytochrome P450 enzymes 1A2 and 3A4.
In vitro studies demonstrated that varenicline does not inhibit human renal transport proteins at therapeutic concentrations. Therefore, drugs that are cleared by renal secretion (e.g., metformin [see below]) are unlikely to be affected by varenicline.
In vitro studies demonstrated the active renal secretion of varenicline is mediated by the human organic cation transporter OCT2. Co-administration with inhibitors of OCT2 (e.g., cimeditine [see below]) may not necessitate a dose adjustment of varenicline tablets as the increase in systemic exposure to varenicline tablets is not expected to be clinically meaningful. Furthermore, since metabolism of varenicline represents less than 10% of its clearance, drugs known to affect the cytochrome P450 system are unlikely to alter the pharmacokinetics of varenicline tablets [see Clinical Pharmacology (12.3)]; therefore, a dose adjustment of varenicline tablets would not be required.
Drug interaction studies were performed with varenicline and digoxin, warfarin, transdermal nicotine, bupropion, cimetidine, and metformin. No clinically meaningful pharmacokinetic drug-drug interactions have been identified.
Metformin
When co-administered to 30 smokers, varenicline (1 mg twice daily) did not alter the steady-state pharmacokinetics of metformin (500 mg twice daily), which is a substrate of OCT2. Metformin had no effect on varenicline steady-state pharmacokinetics.
Cimetidine
Co-administration of an OCT2 inhibitor, cimetidine (300 mg four times daily), with varenicline (2 mg single dose) to 12 smokers increased the systemic exposure of varenicline by 29% (90% CI: 21.5%, 36.9%) due to a reduction in varenicline renal clearance.
Digoxin
Varenicline (1 mg twice daily) did not alter the steady-state pharmacokinetics of digoxin administered as a 0.25 mg daily dose in 18 smokers.
Warfarin
Varenicline (1 mg twice daily) did not alter the pharmacokinetics of a single 25 mg dose of (R, S)-warfarin in 24 smokers. Prothrombin time (INR) was not affected by varenicline. Smoking cessation itself may result in changes to warfarin pharmacokinetics [see Drug Interactions (7.2)].
Use with Other Drugs for Smoking Cessation
Bupropion: Varenicline (1 mg twice daily) did not alter the steady-state pharmacokinetics of bupropion (150 mg twice daily) in 46 smokers [see Drug Interactions (7.1)].
NRT: Although co-administration of varenicline (1 mg twice daily) and transdermal nicotine (21 mg/day) for up to 12 days did not affect nicotine pharmacokinetics, the incidence of adverse reactions was greater for the combination than for NRT alone [see Drug Interactions (7.1)].
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Lifetime carcinogenicity studies were performed in CD-1 mice and Sprague-Dawley rats. There was no evidence of a carcinogenic effect in mice administered varenicline by oral gavage for 2 years at doses up to 20 mg/kg/day (47 times the maximum recommended human daily (MRHD) exposure based on AUC). Rats were administered varenicline (1, 5, and 15 mg/kg/day) by oral gavage for 2 years. In male rats (n = 65 per sex per dose group), incidences of hibernoma (tumor of the brown fat) were increased at the mid dose (1 tumor, 5 mg/kg/day, 23 times the MRHD exposure based on AUC) and maximum dose (2 tumors, 15 mg/kg/day, 67 times the MRHD exposure based on AUC). The clinical relevance of this finding to humans has not been established. There was no evidence of carcinogenicity in female rats.
Mutagenesis
Varenicline was not genotoxic, with or without metabolic activation, in the following assays: Ames bacterial mutation assay; mammalian CHO/HGPRT assay; and tests for cytogenetic aberrations in vivo in rat bone marrow and in vitro in human lymphocytes.
Impairment of Fertility
There was no evidence of impairment of fertility in either male or female Sprague-Dawley rats administered varenicline succinate up to 15 mg/kg/day (67 and 36 times, respectively, the MRHD exposure based on AUC at 1 mg twice daily).
Maternal toxicity, characterized by a decrease in body weight gain, was observed at 15 mg/kg/day. However, a decrease in fertility was noted in the offspring of pregnant rats who were administered varenicline succinate at an oral dose of 15 mg/kg/day. This decrease in fertility in the offspring of treated female rats was not evident at an oral dose of 3 mg/kg/day (9 times the MRHD exposure based on AUC at 1 mg twice daily).
-
14 CLINICAL STUDIES
The efficacy of varenicline tablets in smoking cessation was demonstrated in six clinical trials in which a total of 3659 chronic cigarette smokers (≥10 cigarettes per day) were treated with varenicline tablets. In all clinical studies, abstinence from smoking was determined by patient self-report and verified by measurement of exhaled carbon monoxide (CO≤10 ppm) at weekly visits. Among the varenicline tablets -treated patients enrolled in these studies, the completion rate was 65%. Except for the dose-ranging study (Study 1) and the maintenance of abstinence study (Study 6), patients were treated for 12 weeks and then were followed for 40 weeks post-treatment. Most patients enrolled in these trials were white (79 to 96%). All studies enrolled almost equal numbers of men and women. The average age of patients in these studies was 43 years. Patients on average had smoked about 21 cigarettes per day for an average of approximately 25 years. Patients set a date to stop smoking (target quit date) with dosing starting 1 week before this date.
Seven additional studies evaluated the efficacy of varenicline tablets in patients with cardiovascular disease, in patients with chronic obstructive pulmonary disease [see Clinical Studies (14.7)], in patients instructed to select their quit date within days 8 and 35 of treatment [see Clinical Studies (14.4)], patients with major depressive disorder [see Clinical Studies (14.9)], patients who had made a previous attempt to quit smoking with varenicline tablets, and either did not succeed in quitting or relapsed after treatment [see Clinical Studies (14.6)], in patients without or with a history of psychiatric disorder enrolled in a postmarketing neuropsychiatric safety outcome trial [see Warnings and Precautions (5.1), Clinical Studies (14.10)], and in patients who were not able or willing to quit abruptly and were instructed to quit gradually [see Clinical studies (14.5)].
In all studies, patients were provided with an educational booklet on smoking cessation and received up to 10 minutes of smoking cessation counseling at each weekly treatment visit according to Agency for Healthcare Research and Quality guidelines.
14.1 Initiation of Abstinence
This was a six-week dose-ranging study comparing varenicline tablets to placebo. This study provided initial evidence that varenicline tablets at a total dose of 1 mg per day or 2 mg per day was effective as an aid to smoking cessation.
Study 2
This study of 627 patients compared varenicline tablets 1 mg per day and 2 mg per day with placebo. Patients were treated for 12 weeks (including one-week titration) and then were followed for 40 weeks post-treatment. Varenicline tablets were given in two divided doses daily. Each dose of varenicline tablets was given in two different regimens, with and without initial dose-titration, to explore the effect of different dosing regimens on tolerability. For the titrated groups, dosage was titrated up over the course of one week, with full dosage achieved starting with the second week of dosing. The titrated and nontitrated groups were pooled for efficacy analysis.
Forty-five percent of patients receiving varenicline tablets 1 mg per day (0.5 mg twice daily) and 51% of patients receiving 2 mg per day (1 mg twice daily) had CO-confirmed continuous abstinence during weeks 9 through 12 compared to 12% of patients in the placebo group (Figure 1). In addition, 31% of the 1 mg per day group and 31% of the 2 mg per day group were continuously abstinent from one week after TQD through the end of treatment as compared to 8% of the placebo group.
Study 3
This flexible-dosing study of 312 patients examined the effect of a patient-directed dosing strategy of varenicline tablets or placebo. After an initial one-week titration to a dose of 0.5 mg twice daily, patients could adjust their dosage as often as they wished between 0.5 mg once daily to 1 mg twice daily per day. Sixty-nine percent of patients titrated to the maximum allowable dose at any time during the study. For 44% of patients, the modal dose selected was 1 mg twice daily; for slightly over half of the study participants, the modal dose selected was 1 mg/day or less.
Of the patients treated with varenicline tablets, 40% had CO-confirmed continuous abstinence during weeks 9 through 12 compared to 12% in the placebo group. In addition, 29% of the varenicline tablets group were continuously abstinent from one week after TQD through the end of treatment as compared to 9% of the placebo group.
Study 4 and Study 5
These identical double-blind studies compared varenicline tablets 2 mg per day, bupropion sustained-release (SR) 150 mg twice daily, and placebo. Patients were treated for 12 weeks and then were followed for 40 weeks post-treatment. The varenicline tablets dosage of 1 mg twice daily was achieved using a titration of 0.5 mg once daily for the initial 3 days followed by 0.5 mg twice daily for the next 4 days. The bupropion SR dosage of 150 mg twice daily was achieved using a 3-day titration of 150 mg once daily. Study 4 enrolled 1022 patients and Study 5 enrolled 1023 patients. Patients inappropriate for bupropion treatment or patients who had previously used bupropion were excluded.
In Study 4, patients treated with varenicline tablets had a superior rate of CO-confirmed abstinence during weeks 9 through 12 (44%) compared to patients treated with bupropion SR (30%) or placebo (17%). The bupropion SR quit rate was also superior to placebo. In addition, 29% of the varenicline tablets group were continuously abstinent from one week after TQD through the end of treatment as compared to 12% of the placebo group and 23% of the bupropion SR group.
Similarly in Study 5, patients treated with varenicline tablets had a superior rate of CO-confirmed abstinence during weeks 9 through 12 (44%) compared to patients treated with bupropion SR (30%) or placebo (18%). The bupropion SR quit rate was also superior to placebo. In addition, 29% of the varenicline tablets group were continuously abstinent from one week after TQD through the end of treatment as compared to 11% of the placebo group and 21% of the bupropion SR group.
Figure 1: Continuous Abstinence, Weeks 9 through 12
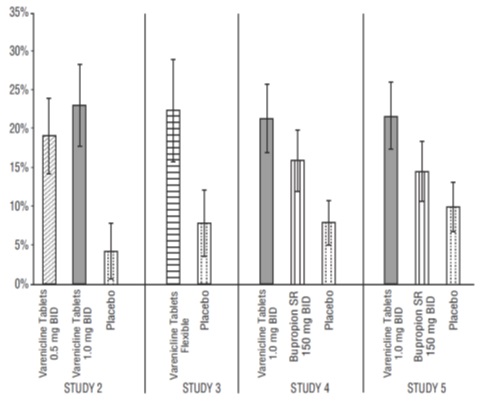
Table 7: Continuous Abstinence, Weeks 9 through 12 (95% confidence interval) BID=twice daily
Varenicline Tablets
0.5 mg BID
Varenicline Tablets
1 mg BID
Varenicline Tablets
Flexible
Bupropion SR
Placebo
Study 2
45%
(39%, 51%)
51%
(44%, 57%)
12%
(6%, 18%)
Study 3
40%
(32%, 48%)
12%
(7%, 17%)
Study 4
44%
(38%, 49%)
30%
(25%, 35%)
17%
(13%, 22%)
Study 5
44%
(38%, 49%)
30%
(25%, 35%)
18%
(14%, 22%)
14.2 Urge to Smoke
Based on responses to the Brief Questionnaire of Smoking Urges and the Minnesota Nicotine Withdrawal scale "urge to smoke" item, varenicline tablets reduced urge to smoke compared to placebo.
14.3 Long-Term Abstinence
Studies 1 through 5 included 40 weeks of post-treatment follow-up. In each study, varenicline tablets -treated patients were more likely to maintain abstinence throughout the follow-up period than were patients treated with placebo (Figure 2, Table 8).
Figure 2: Continuous Abstinence, Weeks 9 through 52
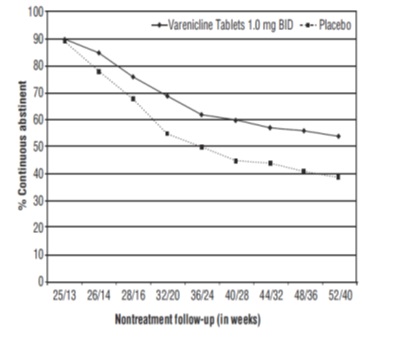
Table 8: Continuous Abstinence, Weeks 9 through 52 (95% confidence interval) Across Different Studies BID=twice daily
Varenicline Tablets 0.5 mg BID
Varenicline Tablets 1 mg BID
Varenicline Tablets Flexible
Bupropion SR
Placebo
Study 2
19%
(14%, 24%)
23%
(18%, 28%)
4%
(1%, 8%)
Study 3
22%
(16%, 29%)
8%
(3%, 12%)
Study 4
21%
(17%, 26%)
16%
(12%, 20%)
8%
(5%, 11%)
Study 5
22%
(17%, 26%)
14%
(11%, 18%)
10%
(7%, 13%)
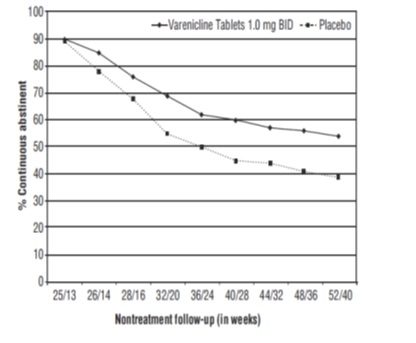
This study assessed the effect of an additional 12 weeks of varenicline tablets therapy on the likelihood of long-term abstinence. Patients in this study (N=1927) were treated with open-label varenicline tablets 1 mg twice daily for 12 weeks. Patients who had stopped smoking for at least a week by Week 12 (N= 1210) were then randomized to double-blind treatment with varenicline tablets (1 mg twice daily) or placebo for an additional 12 weeks and then followed for 28 weeks post-treatment.
The continuous abstinence rate from Week 13 through Week 24 was higher for patients continuing treatment with varenicline tablets (70%) than for patients switching to placebo (50%). Superiority to placebo was also maintained during 28 weeks post-treatment follow-up (varenicline tablets 54% versus placebo 39%).
In Figure 3 below, the x-axis represents the study week for each observation, allowing a comparison of groups at similar times after discontinuation of varenicline tablets; post- varenicline tablets follow-up begins at Week 13 for the placebo group and Week 25 for the varenicline tablets group. The y-axis represents the percentage of patients who had been abstinent for the last week of varenicline tablets treatment and remained abstinent at the given timepoint.
Figure 3: Continuous Abstinence Rate during Nontreatment Follow-Up
14.4 Alternative Instructions for Setting a Quit Date
Varenicline tablets were evaluated in a double-blind, placebo-controlled trial where patients were instructed to select a target quit date between Day 8 and Day 35 of treatment. Subjects were randomized 3:1 to varenicline tablets 1 mg twice daily (N=486) or placebo (N=165) for 12 weeks of treatment and followed for another 12 weeks post- treatment. Patients treated with varenicline tablets had a superior rate of CO-confirmed abstinence during weeks 9 through 12 (54%) compared to patients treated with placebo (19%) and from weeks 9 through 24 (35%) compared to subjects treated with placebo (13%).
14.5 Gradual Approach to Quitting Smoking
Varenicline tablets were evaluated in a 52-week double-blind placebo-controlled study of 1,510 subjects who were not able or willing to quit smoking within four weeks, but were willing to gradually reduce their smoking over a 12 week period before quitting. Subjects were randomized to either varenicline tablets 1 mg twice daily (N=760) or placebo (N=750) for 24 weeks and followed up post-treatment through week 52. Subjects were instructed to reduce the number of cigarettes smoked by at least 50 percent by the end of the first four weeks of treatment, followed by a further 50 percent reduction from week four to week eight of treatment, with the goal of reaching complete abstinence by 12 weeks. After the initial 12-week reduction phase, subjects continued treatment for another 12 weeks. Subjects treated with varenicline tablets had a significantly higher Continuous Abstinence Rate compared with placebo at weeks 15 through 24 (32% vs. 7%) and weeks 15 through 52 (24% vs. 6%).
14.6 Re-Treatment Study
Varenicline tablets were evaluated in a double-blind, placebo-controlled trial of patients who had made a previous attempt to quit smoking with varenicline tablets, and either did not succeed in quitting or relapsed after treatment. Subjects were randomized 1:1 to varenicline tablets 1 mg twice daily (N=249) or placebo (N=245) for 12 weeks of treatment and followed for 40 weeks post-treatment. Patients included in this study had taken varenicline tablets for a smoking-cessation attempt in the past (for a total treatment duration of a minimum of two weeks), at least three months prior to study entry, and had been smoking for at least four weeks.
Patients treated with varenicline tablets had a superior rate of CO-confirmed abstinence during weeks 9 through 12 (45%) compared to patients treated with placebo (12%) and from weeks 9 through 52 (20%) compared to subjects treated with placebo (3%).
Table 9: Continuous Abstinence (95% confidence interval), Re-Treatment Study Weeks 9 through 12
Weeks 9 through 52
Varenicline Tablets
1 mg BID
Placebo
Varenicline Tablets
1 mg BID
Placebo
Retreatment
Study
45%
(39%, 51%)
12%
(8%, 16%)
20%
(15%, 25%)
3%
(1%, 5%)
14.7 Subjects with Chronic Obstructive Pulmonary Disease
Varenicline tablets were evaluated in a randomized, double-blind, placebo-controlled study of subjects aged ≥ 35 years with mild-to-moderate COPD with post-bronchodilator FEV1/FVC <70% and FEV1 ≥ 50% of predicted normal value. Subjects were randomized to varenicline tablets 1 mg twice daily (N=223) or placebo (N=237) for a treatment of 12 weeks and then were followed for 40 weeks post-treatment. Subjects treated with varenicline tablets had a superior rate of CO-confirmed abstinence during weeks 9 through 12 (41%) compared to subjects treated with placebo (9%) and from week 9 through 52 (19%) compared to subjects treated with placebo (6%).
Table 10: Continuous Abstinence (95% confidence interval), Studies in Patients with Chronic Obstructive Pulmonary Disease (COPD) Weeks 9 through 12
Weeks 9 through 52
V arenicline Tablets
1 mg BID
Placebo
Varenicline Tablets
1 mg BID
Placebo
COPD Study
41%
(34%, 47%)
9%
(6%, 13%)
19%
(14%, 24%)
6%
(3%, 9%)
14.8 Subjects with Cardiovascular Disease and Other Cardiovascular Analyses
Varenicline tablets were evaluated in a randomized, double-blind, placebo-controlled study of subjects aged 35 to 75 years with stable, documented cardiovascular disease (diagnoses other than, or in addition to, hypertension) that had been diagnosed for more than 2 months. Subjects were randomized to varenicline tablets 1 mg twice daily (N=353) or placebo (N=350) for a treatment period of 12 weeks and then were followed for 40 weeks post-treatment. Subjects treated with varenicline tablets had a superior rate of CO-confirmed abstinence during weeks 9 through 12 (47%) compared to subjects treated with placebo (14%) and from week 9 through 52 (20%) compared to subjects treated with placebo (7%).
Table 11:Continuous Abstinence (95% confidence interval), Studies in Patients with Cardiovascular Disease (CVD) Weeks 9 through 12
Weeks 9 through 52
Varenicline Tablets
1 mg BID
Placebo
Varenicline Tablets
1 mg BID
Placebo
CVD Study
47%
(42%, 53%)
14%
(11%, 18%)
20%
(16%, 24%)
7%
(5%, 10%)
In this study, all-cause and CV mortality was lower in patients treated with varenicline tablets, but certain nonfatal CV events occurred more frequently in patients treated with varenicline tablets than in patients treated with placebo [see Warnings and Precautions (5.5), Adverse Reactions (6.1)]. Table 12 below shows mortality and the incidence of selected nonfatal serious CV events occurring more frequently in the varenicline tablets arm compared to the placebo arm. These events were adjudicated by an independent blinded committee. Nonfatal serious CV events not listed occurred at the same incidence or more commonly in the placebo arm. Patients with more than one CV event of the same type are counted only once per row. Some of the patients requiring coronary revascularization underwent the procedure as part of management of nonfatal MI and hospitalization for angina.
Table 12: Mortality and Adjudicated Nonfatal Serious Cardiovascular Events in the Placebo-Controlled Varenicline Tablets Trial in Patients with Stable Cardiovascular Disease Mortality and Cardiovascular Events
Varenicline Tablets
(N=353)
n (%)
Placebo
(N=350)
n (%)
Mortality (Cardiovascular and All-cause up to 52 weeks)
Cardiovascular
1 (0.3)
2 (0.6)
All-cause
2 (0.6)
5 (1.4)
Nonfatal Cardiovascular Events (rate on Varenicline Tablets> Placebo)
Up to 30 days after treatment
Nonfatal myocardial infarction
4 (1.1)
1 (0.3)
Nonfatal Stroke
2 (0.6)
0 (0)
Beyond 30 days after treatment and up to 52 weeks
Nonfatal myocardial infarction
3 (0.8)
2 (0.6)
Need for coronary revascularization
7 (2.0)
2 (0.6)
Hospitalization for angina pectoris
6 (1.7)
4 (1.1)
Transient ischemia attack
1 (0.3)
0 (0)
New diagnosis of peripheral vascular disease (PVD) or admission for a PVD procedure
5 (1.4)
2 (0.6)
Following the CVD study, a meta-analysis of 15 clinical trials of ≥12 weeks treatment duration, including 7002 patients (4190 varenicline tablets, 2812 placebo), was conducted to systematically assess the CV safety of varenicline tablets. The study in patients with stable CV disease described above was included in the meta-analysis. There were lower rates of all-cause mortality (varenicline tablets 6 [0.14%]; placebo 7 [0.25%]) and CV mortality (varenicline tablets 2 [0.05%]; placebo 2 [0.07%]) in the varenicline tablets arms compared with the placebo arms in the meta-analysis.
The key CV safety analysis included occurrence and timing of a composite endpoint of Major Adverse Cardiovascular Events (MACE), defined as CV death, nonfatal MI, and nonfatal stroke. These events included in the endpoint were adjudicated by a blinded, independent committee. Overall, a small number of MACE occurred in the trials included in the meta-analysis, as described in Table 13. These events occurred primarily in patients with known CV disease.
Table 13: Number of MACE cases, Hazard Ratio and Rate Difference in a Meta-Analysis of 15 Clinical Trials Comparing Varenicline Tablets to Placebo* *Includes MACE occurring up to 30 days post-treatment.
Varenicline Tablets
N=4190
Placebo
N=2812
MACE cases, n (%)
13 (0.31%)
6 (0.21%)
Patient-years of exposure
1316
839
Hazard Ratio (95% CI)
1.95 (0.79, 4.82)
Rate Difference per 1,000 patient-years (95% CI)
6.30 (-2.40, 15.10)
The meta-analysis showed that exposure to varenicline tablets resulted in a hazard ratio for MACE of 1.95 (95% confidence interval from 0.79 to 4.82) for patients up to 30 days after treatment; this is equivalent to an estimated increase of 6.3 MACE events per 1,000 patient-years of exposure. The meta-analysis showed higher rates of CV endpoints in patients on varenicline tablets relative to placebo across different time frames and pre-specified sensitivity analyses, including various study groupings and CV outcomes. Although these findings were not statistically significant they were consistent. Because the number of events was small overall, the power for finding a statistically significant difference in a signal of this magnitude is low.
Additionally, a cardiovascular endpoint analysis was added to the postmarketing neuropsychiatric safety outcome study along with a non-treatment extension, [see Warnings and Precautions (5.5), Adverse Reactions (6.1), Clinical Studies (14.10)].
14.9 Subjects with Major Depressive Disorder
Varenicline tablets were evaluated in a randomized, double-blind, placebo-controlled study of subjects aged 18 to 75 years with major depressive disorder without psychotic features (DSM-IV TR). If on medication, subjects were to be on a stable antidepressant regimen for at least two months. If not on medication, subjects were to have experienced a major depressive episode in the past 2 years, which was successfully treated. Subjects were randomized to varenicline tablets 1 mg twice daily (N=256) or placebo (N=269) for a treatment of 12 weeks and then followed for 40 weeks post-treatment. Subjects treated with varenicline tablets had a superior rate of CO-confirmed abstinence during weeks 9 through 12 (36%) compared to subjects treated with placebo (16%) and from week 9 through 52 (20%) compared to subjects treated with placebo (10%).
Table 14: Continuous Abstinence (95% confidence interval), Study in Patients with Major Depressive Disorder (MDD) BID = twice daily
Weeks 9 through 12
Weeks 9 through 52
Varenicline Tablets
1 mg BID
Placebo
Varenicline Tablets
1 mg BID
Placebo
MDD Study
36%
(30%, 42%)
16%
(11%, 20%)
20%
(15%, 25%)
10%
(7%, 14%)
14.10 Postmarketing Neuropsychiatric Safety Outcome Trial
Varenicline tablets were evaluated in a randomized, double-blind, active and placebo-controlled trial that included subjects without a history of psychiatric disorder (non-psychiatric cohort, N=3912) and with a history of psychiatric disorder (psychiatric cohort, N=4003). Subjects aged 18-75 years, smoking 10 or more cigarettes per day were randomized 1:1:1:1 to varenicline tablets 1 mg BID, bupropion SR 150 mg BID, NRT patch 21 mg/day with taper or placebo for a treatment period of 12 weeks; they were then followed for another 12 weeks post-treatment. [See Warnings and Precautions (5.1)]
A composite safety endpoint intended to capture clinically significant neuropsychiatric (NPS) adverse events included the following NPS adverse events: anxiety, depression, feeling abnormal, hostility, agitation, aggression, delusions, hallucinations, homicidal ideation, mania, panic, paranoia, psychosis, irritability, suicidal ideation, suicidal behavior or completed suicide.
As shown in Table 15, the use of varenicline tablets, bupropion, and NRT in the non-psychiatric cohort was not associated with an increased risk of clinically significant NPS adverse events compared with placebo. Similarly, in the non-psychiatric cohort, the use of varenicline tablets was not associated with an increased risk of clinically significant NPS adverse events in the composite safety endpoint compared with bupropion or NRT.
Table 15: Number of Patients with Clinically Significant or Serious NPS Adverse Events by Treatment Group Among Patients without a History of Psychiatric Disorder Varenicline Tablets (N=975)
n (%)
Bupropion
(N=968)
n (%)
NRT
(N=987)
n (%)
Placebo (N=982)
n (%)
Clinically Significant NPS
30 (3.1)
34 (3.5)
33 (3.3)
40 (4.1)
Serious NPS
1 (0.1)
5 (0.5)
1 (0.1)
4 (0.4)
Psychiatric Hospitalizations
1 (0.1)
2 (0.2)
0 (0.0)
1 (0.1)
As shown in Table 16, there were more clinically significant NPS adverse events reported in patients in the psychiatric cohort in each treatment group compared with the non-psychiatric cohort. The incidence of events in the composite endpoint was higher for each of the active treatments compared to placebo: Risk Differences (RDs) (95%CI) vs placebo were 2.7% (-0.05, 5.4) for varenicline tablets, 2.2% (-0.5, 4.9) for bupropion, and 0.4% (-2.2, 3.0) for NRT transdermal nicotine.
Table 16: Number of Patients with Clinically Significant or Serious NPS Adverse Events by Treatment Group Among Patients with a History of Psychiatric Disorder Varenicline Tablets
(N=1007)
n (%)
Bupropion
(N=1004)
n (%)
NRT (N=995)
n (%)
Placebo (N=997)
n (%)
Clinically Significant NPS
123 (12.2)
118 (11.8)
98 (9.8)
95 (9.5)
Serious NPS
6 (0.6)
8 (0.8)
4 (0.4)
6 (0.6)
Psychiatric hospitalizations
5 (0.5)
8 (0.8)
4 (0.4)
2 (0.2)
There was one completed suicide, which occurred during treatment in a patient treated with placebo in the non-psychiatric cohort. There were no completed suicides reported in the psychiatric cohort.
In both cohorts, subjects treated with varenicline tablets had a superior rate of CO-confirmed abstinence during weeks 9 through 12 and 9 through 24 compared to subjects treated with bupropion, nicotine patch and placebo.
Table 17: Continuous Abstinence (95% confidence interval), Study in Patients with or without a History of Psychiatric Disorder
Varenicline Tablets
1 mg BID
Bupropion SR
150 mg BID
NRT
21 mg/day with taper
Placebo
Weeks 9 through 12
Non-Psychiatric
Cohort
38%
(35%, 41%)
26%
(23%, 29%)
26%
(24%, 29%)
14%
(12%, 16%)
Psychiatric Cohort
29%
(26%, 32%)
19%
(17%, 22%)
20%
(18%, 23%)
11%
(10%, 14%)
Weeks 9 through 24
Non-Psychiatric
Cohort
25%
(23%, 28%)
19%
(16%, 21%)
18%
(16%, 21%)
11%
(9%, 13%)
Psychiatric Cohort
18%
(16%, 21%)
14%
(12%, 16%)
13%
(11%, 15%)
8%
(7%, 10%)
Cardiovascular Outcome Analysis
To obtain another source of data regarding the CV risk of varenicline tablets, a cardiovascular endpoint analysis was added to the postmarketing neuropsychiatric safety outcome study along with a non-treatment extension. In the parent study (N=8027), subjects aged 18-75 years, smoking 10 or more cigarettes per day were randomized 1:1:1:1 to varenicline tablets 1 mg BID, bupropion SR 150 mg BID, nicotine replacement therapy (NRT) patch 21 mg/day or placebo for a treatment period of 12 weeks; they were then followed for another 12 weeks post-treatment. The extension study enrolled 4590 (57.2%) of the 8027 subjects who were randomized and treated in the parent study and followed them for additional 28 weeks. Of all treated subjects, 1743 (21.7%) had a medium CV risk and 640 (8.0%) had a high CV risk, as defined by Framingham score. Note that one site from the parent study was excluded in the assessment of CV safety and two sites were excluded in the assessment of neuropsychiatric safety.
The primary CV endpoint was the time to major adverse CV event (MACE), defined as CV death, nonfatal myocardial infarction or nonfatal stroke during treatment.
Deaths and CV events were adjudicated by a blinded, independent committee. Table 18 below shows the incidence of MACE and Hazard Ratios compared to placebo for all randomized subjects exposed to at least 1 partial dose of study treatment in the parent study.
Table 18: The Incidence of MACE and Hazard Ratios in the Cardiovascular Safety Assessment Trial in Subjects without or with a History of Psychiatric Disorder [IR] indicates incidence rate per 1000 person-years
*during treatment in the parent neuropsychiatric safety study
**either the end of the extension study or the end of parent neuropsychiatric safety study for those subjects not enrolled into the extension study
Varenicline Tablets
N=2006
Bupropion
N=1997
NRT
N=2017
Placebo
N=2007
During treatment*
MACE, n [IR]
1 [2.4]
2 [4.9]
1 [2.4]
4 [9.8]
Hazard Ratio (95% CI)
vs. placebo
0.24
(0.03, 2.18)
0.49
(0.09, 2.69)
0.24
(0.03, 2.18)
Through end of study**
MACE, n [IR]
3 [2.1]
9 [6.3]
6 [4.3]
8 [5.7]
Hazard Ratio (95% CI)
vs. placebo
0.36
(0.10, 1.36)
1.09
(0.42, 2.83)
0.74
(0.26, 2.13)
For this study, MACE+ was defined as any MACE or a new onset or worsening peripheral vascular disease (PVD) requiring intervention, a need for coronary revascularization, or hospitalization for unstable angina. Incidence rates of MACE+ and all-cause mortality for all randomized subjects exposed to at least 1 partial dose of study treatment in the parent study are shown for all treatment groups during treatment, and through end of study in the Table 19 below.
Table 19: The Incidence of MACE+ and All-Cause Death in the Cardiovascular Safety Assessment Trial in Subjects without or with a History of Psychiatric Disorder [IR] indicates incidence rate per 1000 person-years
*during treatment in the parent neuropsychiatric safety study
**either the end of the extension study or the end of the parent neuropsychiatric safety study for those subjects not enrolled into the extension study
Varenicline Tablets
N=2006
Bupropion
N=1997
NRT
N=2017
Placebo
N=2007
During treatment*
MACE+, n [IR]
5 [12.1]
4 [9.9]
2 [4.8]
5 [12.2]
All-cause deaths, n [IR]
0
2 [4.9]
0
2 [4.9]
Through end of study**
MACE+, n [IR]
10 [6.9]
15 [10.5]
10 [7.1]
12 [8.6]
All-cause deaths, n [IR]
2 [1.4]
4 [2.8]
3 [2.1]
4 [2.9]
The number of subjects who experienced MACE, MACE+ and all-cause death was similar or lower among patients treated with varenicline tablets than patients treated with placebo. The number of events observed overall was too low to distinguish meaningful differences between the treatment arms.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Varenicline tablets are supplied for oral administration in two strengths: 0.5 mg white to off white, round, biconvex-coated tablets, debossed with "T" on one side and "2" on the other side and 1 mg yellow, round, biconvex-coated tablets, debossed with "T" on one side and "1" on the other side. Varenicline tablets are supplied in the following package configurations:
Description
NDC
Packs
Starting 4-week card: 0.5 mg x 11 Tablets and 1 mg x 42 Tablets
NDC 70748 -125-11
Continuing 4-week card: 1 mg x 56 Tablets
NDC 70748 -126-11
Starting Month Box: 0.5 mg x 11 Tablets and 1 mg x 42 Tablets
NDC 70748 -125-13
Continuing Month Box: 1 mg x 56 Tablets
NDC 70748 -126-13
Bottles
0.5 mg - Bottle of 56 Tablets
NDC 70748 -127-49
1 mg - Bottle of 56 Tablets
NDC 70748 -128-49
Store at 25ºC (77ºF); excursions permitted to 15ºC to 30ºC (59ºF to 86ºF) (see USP Controlled Room Temperature).
-
17 PATIENT COUNSELING INFORMATION
See FDA-approved patient labeling (Medication Guide)
Initiate Treatment and Continue to Attempt to Quit if Lapse
Instruct patients to set a date to quit smoking and to initiate varenicline tablets treatment one week before the quit date. Alternatively, the patient can begin varenicline tablets dosing and then set a date to quit smoking between days 8 and 35 of treatment. Encourage patients to continue to attempt to quit if they have early lapses after quit day [see Dosage and Administration (2.1)].
For patients who are sure that they are not able or willing to quit abruptly, a gradual approach to quitting smoking with varenicline tablets may be considered. Patients should begin varenicline tablets dosing and reduce smoking during the first 12 weeks of treatment, then quit by the end of that period and continue treatment for an additional 12 weeks for a total of 24 weeks [see Dosage and Administration (2.1)].
Encourage patients who are motivated to quit and who did not succeed in stopping smoking during prior varenicline tablets therapy for reasons other than intolerability due to adverse events, or who relapsed after treatment to make another attempt with varenicline tablets once factors contributing to the failed attempt have been identified and addressed [see Dosage and Administration (2.1), Clinical Studies (14.6)].
How to Take
Advise patients that varenicline tablets should be taken orally after eating, and with a full glass of water [see Dosage and Administration (2.1)].
Starting Week Dosage
Instruct patients on how to titrate varenicline tablets, beginning at a dose of 0.5 mg/day. Explain that one 0.5 mg tablet should be taken daily for the first three days, and that for the next four days, one 0.5 mg tablet should be taken in the morning and one 0.5 mg tablet should be taken in the evening [see Dosage and Administration (2.1)].
Continuing Weeks Dosage
Advise patients that, after the first seven days, the dose should be increased to one 1 mg tablet in the morning and one 1 mg tablet in the evening [see Dosage and Administration (2.1)].
Dosage Adjustment for varenicline tablets or Other Drugs
Inform patients that nausea and insomnia are side effects of varenicline tablets and are usually transient; however, advise patients that if they are persistently troubled by these symptoms, they should notify the prescribing physician so that a dose reduction can be considered.
Inform patients that some drugs may require dose adjustment after quitting smoking [see Dosage and Administration (2.1)].
Counseling and Support
Provide patients with educational materials and necessary counseling to support an attempt at quitting smoking [see Dosage and Administration (2.1)].
Neuropsychiatric Adverse Events
Inform patients that some patients have experienced changes in mood (including depression and mania), psychosis, hallucinations, paranoia, delusions, homicidal ideation, aggression, hostility, agitation, anxiety, and panic, as well as suicidal ideation and suicide when attempting to quit smoking while taking varenicline tablets. Instruct patients to discontinue varenicline tablets and contact a healthcare professional if they experience such symptoms [see Warnings and Precautions (5.1), Adverse Reactions (6.2)].
History of Psychiatric Illness
Encourage patients to reveal any history of psychiatric illness prior to initiating treatment.
Nicotine Withdrawal
Inform patients that quitting smoking, with or without varenicline tablets, may be associated with nicotine withdrawal symptoms (including depression or agitation) or exacerbation of pre-existing psychiatric illness.
Seizures
Encourage patients to report any history of seizures or other factors that can lower seizure threshold. Instruct patients to discontinue varenicline tablets and contact a healthcare provider immediately if they experience a seizure while on treatment [see Warnings and Precautions (5.2)].
Interaction with Alcohol
Advise patients to reduce the amount of alcohol they consume while taking varenicline tablets until they know whether varenicline tablets affect their tolerance for alcohol [see Warnings and Precautions (5.3), Adverse Reactions (6.2)].
Driving or Operating Machinery
Advise patients to use caution driving or operating machinery until they know how quitting smoking and/or varenicline may affect them [see Warnings and Precautions (5.4)].
Cardiovascular Events
Patients should be instructed to notify their healthcare providers of symptoms of new or worsening cardiovascular events and to seek immediate medical attention if they experience signs and symptoms of myocardial infarction or stroke [see Warnings and Precautions (5.5), Adverse Reactions (6.1)].
Somnambulism
Patients should be instructed to discontinue varenicline tablets and notify their healthcare providers if they experience somnambulism [see Warnings and Precautions (5.6)].
Angioedema
Inform patients that there have been reports of angioedema, with swelling of the face, mouth (lip, gum, tongue) and neck (larynx and pharynx) that can lead to life-threatening respiratory compromise. Instruct patients to discontinue varenicline tablets and immediately seek medical care if they experience these symptoms [see Warnings and Precautions (5.7), Adverse Reactions (6.2)].
Serious Skin Reactions
Inform patients that serious skin reactions, such as Stevens-Johnson Syndrome and erythema multiforme, were reported by some patients taking varenicline tablets. Advise patients to stop taking varenicline tablets at the first sign of rash with mucosal lesions or skin reaction and contact a healthcare provider immediately [see Warnings and Precautions (5.8), Adverse Reactions (6.2)].
Vivid, Unusual, or Strange Dreams
Inform patients that they may experience vivid, unusual or strange dreams during treatment with varenicline tablets.
Pregnancy and Lactation
Patients who are pregnant or breastfeeding or planning to become pregnant should be advised of: the risks of smoking to a pregnant mother and her developing baby, the potential risks of varenicline tablets use during pregnancy and breastfeeding, and the benefits of smoking cessation with and without varenicline tablets. Advise breastfeeding women to monitor the infant for seizures and vomiting [see Use in Specific Populations (8.1 and 8.2)].
- SPL UNCLASSIFIED SECTION
-
MEDICATION GUIDE
Varenicline (va-ren-i-cline) Tablets
What is the most important information I should know about varenicline tablets?
When you try to quit smoking, with or without varenicline tablets, you may have symptoms that may be due to nicotine withdrawal, including:
- urge to smoke
- frustration
- restlessness
- depressed mood
- anger
- decreased heart rate
- trouble sleeping
- feeling anxious
- increased appetite
- irritability
- difficulty concentrating
- weight gain
Some people have even experienced suicidal thoughts when trying to quit smoking without medication.
Sometimes quitting smoking can lead to worsening of mental health problems that you already have, such as depression.
Some people have had serious side effects while taking varenicline tablets to help them quit smoking, including:
New or worse mental health problems, such as changes in behavior or thinking, aggression, hostility, agitation, depressed mood, or suicidal thoughts or actions. Some people had these symptoms when they began taking varenicline tablets, and others developed them after several weeks of treatment, or after stopping varenicline tablets. These symptoms happened more often in people who had a history of mental health problems before taking varenicline tablets, than in people without a history of mental health problems.
Stop taking varenicline tablets and call your healthcare provider right away if you, your family, or caregiver notice any of these symptoms. Work with your healthcare provider to decide whether you should continue to take varenicline tablets. In many people, these symptoms went away after stopping varenicline tablets, but in some people symptoms continued after stopping varenicline tablets. It is important for you to follow-up with your healthcare provider until your symptoms go away.
Before taking varenicline tablets, tell your healthcare provider if you have ever had depression or other mental health problems. You should also tell your healthcare provider about any symptoms you had during other times you tried to quit smoking, with or without varenicline tablets.
What are varenicline tablets?
Varenicline tablets are prescription medicine to help people stop smoking.
Quitting smoking can lower your chances of having lung disease, heart disease or getting certain types of cancer that are related to smoking.
Varenicline tablets have not been shown to be effective in children 16 years of age and under. Varenicline tablets should not be used in children 16 years of age and under.
It is not known if varenicline tablets are safe and effective when used with other stop smoking medicines.
Who should not take varenicline tablets?
Do not take varenicline tablets if you have had a serious allergic or skin reaction to varenicline tablets. Symptoms may include:
- swelling of the face, mouth (tongue, lips, gums), throat or neck
- trouble breathing
- rash, with peeling skin
- blisters in your mouth
What should I tell my healthcare provider before taking varenicline tablets?
See "What is the most important information I should know about varenicline tablets?"
Before you take varenicline tablets, tell your healthcare provider if you:
- use other treatments to quit smoking. Using varenicline tablets with a nicotine patch may cause nausea, vomiting, headache, dizziness, upset stomach, and tiredness to happen more often than if you just use a nicotine patch alone.
- have kidney problems or get kidney dialysis. Your healthcare provider may prescribe a lower dose of varenicline tablets for you.
- have a history of seizures
- drink alcohol
- have heart or blood vessel problems
- have any other medical conditions
- are pregnant or plan to become pregnant.
- are breastfeeding. It is not known if varenicline passes into breast milk. If you breastfeed and take varenicline tablets, monitor your baby for seizures as well as spitting up or vomiting more than normal.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins and herbal supplements. Your healthcare provider may need to change the dose of some of your medicines when you stop smoking.
You should not use varenicline tablets while using other medicines to quit smoking. Tell your healthcare provider if you use other treatments to quit smoking.
Know the medicines you take. Keep a list of them with you to show your healthcare provider and pharmacist when you get a new medicine.
How should I take varenicline tablets?
- There are 3 ways that you can use varenicline tablets to help you quit smoking. Talk to your healthcare provider about the following 3 ways to use varenicline tablets:
- Choose a quit date when you will stop smoking. Start taking varenicline tablets 1 week (7 days) before your quit date . Take varenicline tablets for 12 weeks. OR
- Start taking varenicline tablets before you choose a quit date . Pick a date to quit smoking that is between days 8 and 35 of treatment. Take varenicline tablets for 12 weeks. OR
- If you are sure that you are not able or willing to quit smoking right away, start taking varenicline tablets and reduce smoking during the first 12 weeks of treatment, as follows:
Weeks 1 through 4
Reduce your smoking to reach one-half of your starting daily number of cigarettes.
Example: If you usually smoke 20 cigarettes each day, reduce your smoking to 10 cigarettes each day during weeks 1 through 4.
Weeks 5 through 8
Reduce your smoking to reach one-quarter of your starting daily number of cigarettes.
Example: If you usually smoked 20 cigarettes each day, reduce your smoking to 5 cigarettes each day during weeks 5 through 8.
Weeks 9 through 12
Keep reducing your smoking until you are no longer smoking (you reach zero cigarettes each day).
Aim to quit by the end of the 12th week of treatment, or sooner if you feel ready. Continue to take varenicline tablets for another 12 weeks, for a total of 24 weeks of treatment.
Starting varenicline tablets before your quit date gives varenicline tablets time to build up in your body. You can keep smoking during this time. Take varenicline tablets exactly as prescribed by your healthcare provider.
- Varenicline tablets come as a white tablet (0.5 mg) and a yellow tablet (1 mg). You start with the white tablet and then usually go to the yellow tablet. See the chart below for dosing instructions for adults.
Day 1 to Day 3
o White tablet (0.5 mg)
o Take 1 tablet each day
Day 4 to Day 7
o White tablet (0.5 mg)
o Take 1 in the morning and 1 in the evening
Day 8 to end of treatment
o Yellow tablet (1 mg)
o Take 1 in the morning and 1 in the evening
- Make sure that you try to stop smoking on your quit date. If you slip-up and smoke, try again. Some people need to take varenicline tablets for a few weeks for varenicline tablets to work best.
- Most people will take varenicline tablets for up to 12 weeks. If you have completely quit smoking by 12 weeks, your healthcare provider may prescribe varenicline tablets for another 12 weeks to help you stay cigarette-free.
- Take varenicline tablets after eating and with a full glass (8 ounces) of water.
- This dosing schedule may not be right for everyone. Talk to your healthcare provider if you are having side effects such as nausea, strange dreams, or sleep problems. Your healthcare provider may want to reduce your dose.
- If you miss a dose of varenicline tablets, take it as soon as you remember. If it is almost time for your next dose, skip the missed dose. Just take your next dose at your regular time.
What should I avoid while taking varenicline tablets?
- Use caution when driving or operating machinery until you know how varenicline tablets affect you. Varenicline tablets may make you feel sleepy, dizzy, or have trouble concentrating, making it hard to drive or perform other activities safely.
- Decrease the amount of alcoholic beverages that you drink during treatment with varenicline tablets until you know if varenicline tablets affect your ability to tolerate alcohol. Some people have experienced the following when drinking alcohol during treatment with varenicline tablets:
- increased drunkenness (intoxication)
- unusual or sometimes aggressive behavior
- no memory of things that have happened
What are the possible side effects of varenicline tablets?
Serious side effects of varenicline tablets may include:
- See "What is the most important information I should know about varenicline tablets?"
- Seizures. Some people have had seizures during treatment with varenicline tablets. In most cases, the seizures have happened during the first month of treatment with varenicline tablets. If you have a seizure during treatment with varenicline tablets, stop taking varenicline tablets and contact your healthcare provider right away.
- New or worse heart or blood vessel (cardiovascular) problems, mostly in people, who already have cardiovascular problems. Tell your healthcare provider if you have any changes in symptoms during treatment with varenicline tablets.
Get emergency medical help right away if you have any of the following symptoms of a heart attack, including:
- chest discomfort (uncomfortable pressure, squeezing, fullness or pain) that lasts more than a few minutes, or that goes away and comes back
- pain or discomfort in one or both arms, back, neck, jaw or stomach
- shortness of breath, sweating, nausea, vomiting, or feeling lightheaded associated with chest discomfort
- Sleepwalking can happen with varenicline tablets, and can sometimes lead to behavior that is harmful to you or other people, or to property. Stop taking varenicline tablets and tell your healthcare provider if you start sleepwalking.
- Allergic reactions can happen with varenicline tablets. Some of these allergic reactions can be life-threatening.
- Serious skin reactions, including rash, swelling, redness, and peeling of the skin. Some of these skin reactions can become life-threatening.
Stop taking varenicline tablets and get medical help right away if you have any of the following symptoms:
- swelling of the face, mouth (tongue, lips, and gums), throat or neck
- trouble breathing
- rash with peeling skin
- blisters in your mouth
The most common side effects of varenicline tablets include:
- nausea
- sleep problems (trouble sleeping or vivid, unusual, or strange dreams)
- constipation
- gas
- vomiting
Tell your healthcare provider about side effects that bother you or that do not go away.
These are not all the side effects of varenicline tablets. Ask your healthcare provider or pharmacist for more information.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA 1088. You may also report side effects to Lupin Pharmaceuticals, Inc. at 1-800-399-2561.
How should I store varenicline tablets?
- Store varenicline tablets at room temperature between 68ºF to 77ºF (20ºC to 25ºC)
- Keep varenicline tablets and all medicines out of the reach of children.
General information about the safe and effective use of varenicline tablets
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use varenicline tablets for a condition for which it was not prescribed. Do not give your varenicline tablets to other people, even if they have the same symptoms that you have. It may harm them. If you would like more information, talk with your healthcare provider. You can ask your healthcare provider or pharmacist for information about varenicline tablets that is written for healthcare professionals.
If you are motivated to quit smoking and did not succeed during prior varenicline tablets treatment for reasons other than side effects, or if you returned to smoking after treatment, speak with your healthcare provider about whether another course of varenicline tablets therapy may be right for you.
What are the ingredients in varenicline tablets?
Active ingredient: varenicline tartrate
Inactive ingredients: anhydrous dibasic calcium phosphate, croscarmellose sodium, hypromellose, magnesium stearate, maltodextrin, talc and titanium dioxide. The 1 mg tablet also contains iron oxide yellow.
This Medication Guide has been approved by the U.S. Food and Drug Administration.
Manufactured for:
Lupin Pharmaceuticals, Inc.
Baltimore, Maryland 21202
United States.
Manufactured by:
Lupin Limited
Pithampur (M.P.) 454 775, INDIA
Revised: May 2023
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
0.5 mg
NDC 70748-127-49
Bottle Label -0.5 mg - 56s Tablets
Rx only
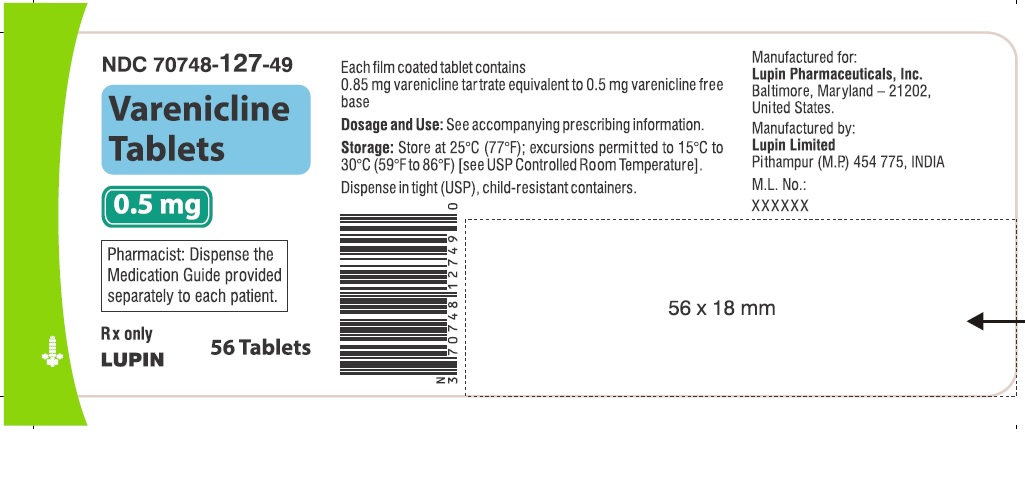
1 mg
NDC 70748-128-49
Bottle Label -1 mg - 56s Tablets
Rx only

NDC 70748-125-11
Starting 4-Week Pack-Wallet Label
Rx only
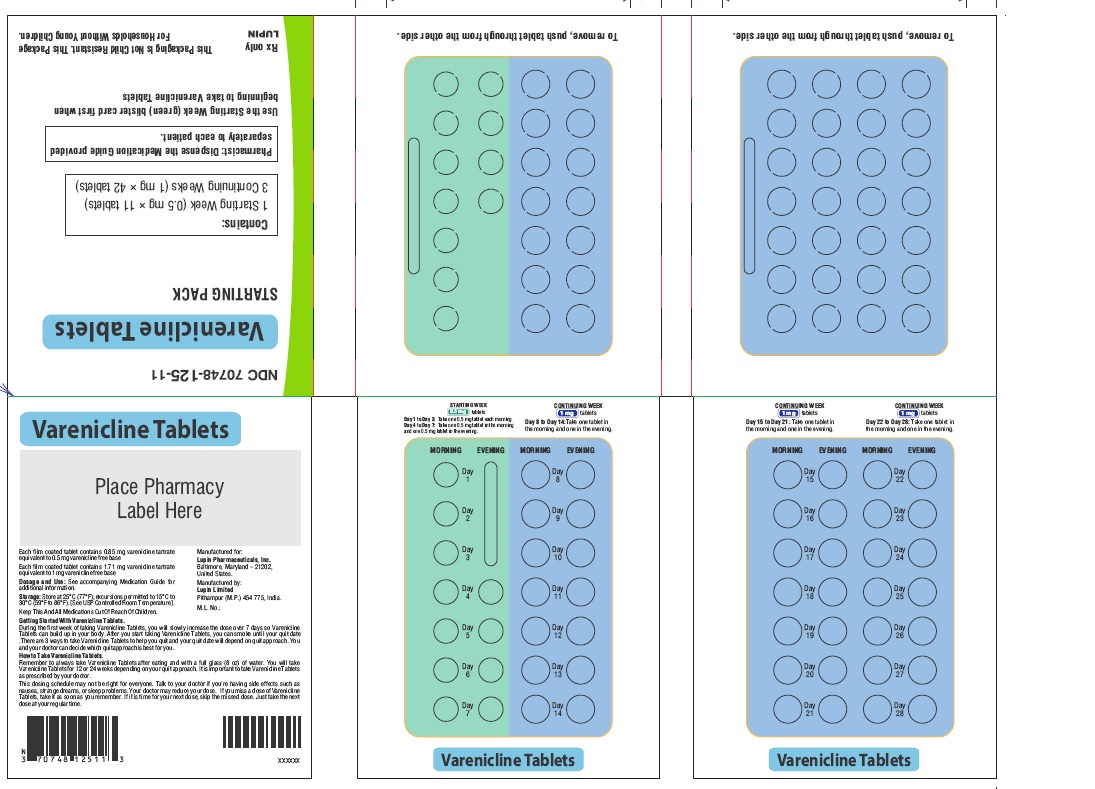
1 mg
NDC 70748-126-11
Continuing 4-Week Pack-Wallet Label
Rx only

NDC 70748-125-11
Starting 4-Week Pack-Pouch Label
Rx only
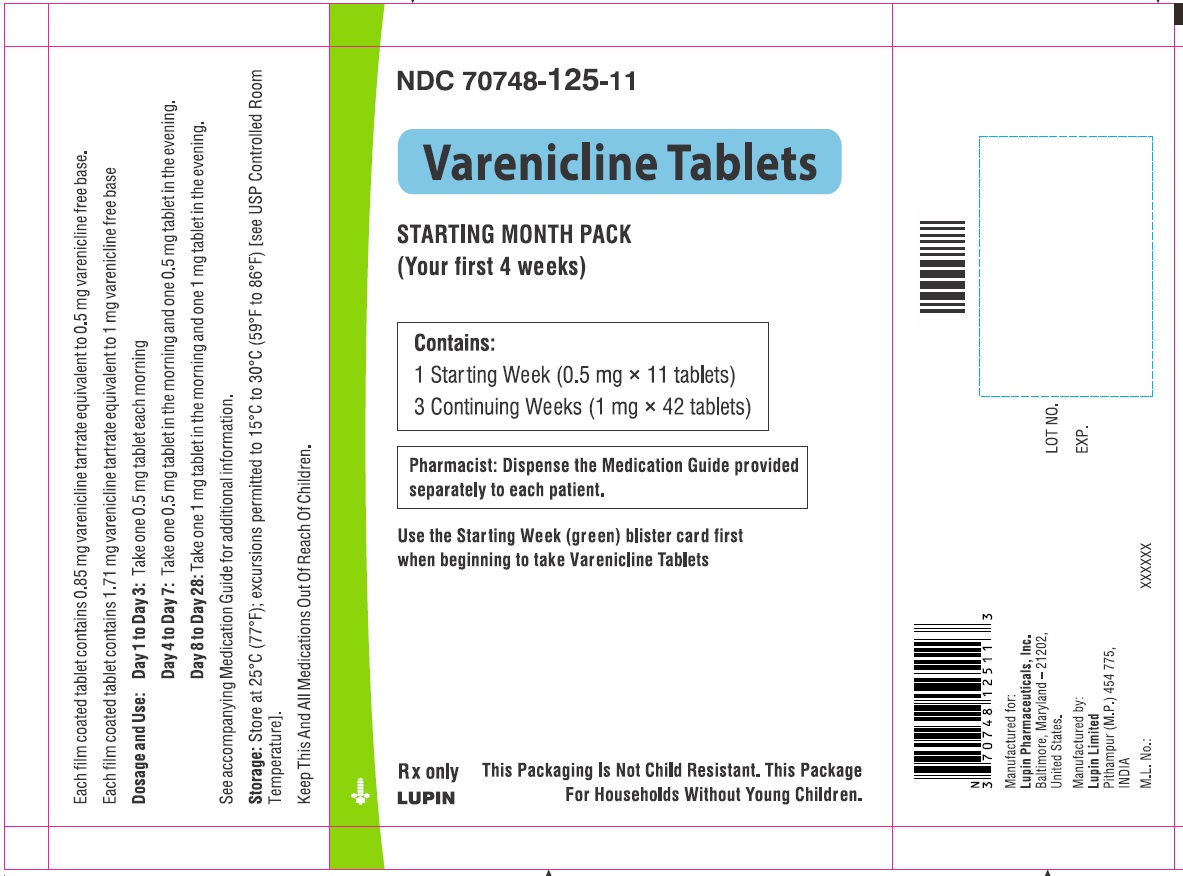
NDC 70748-126-11
Continuing 4-Week Pack-Pouch Label
Rx only
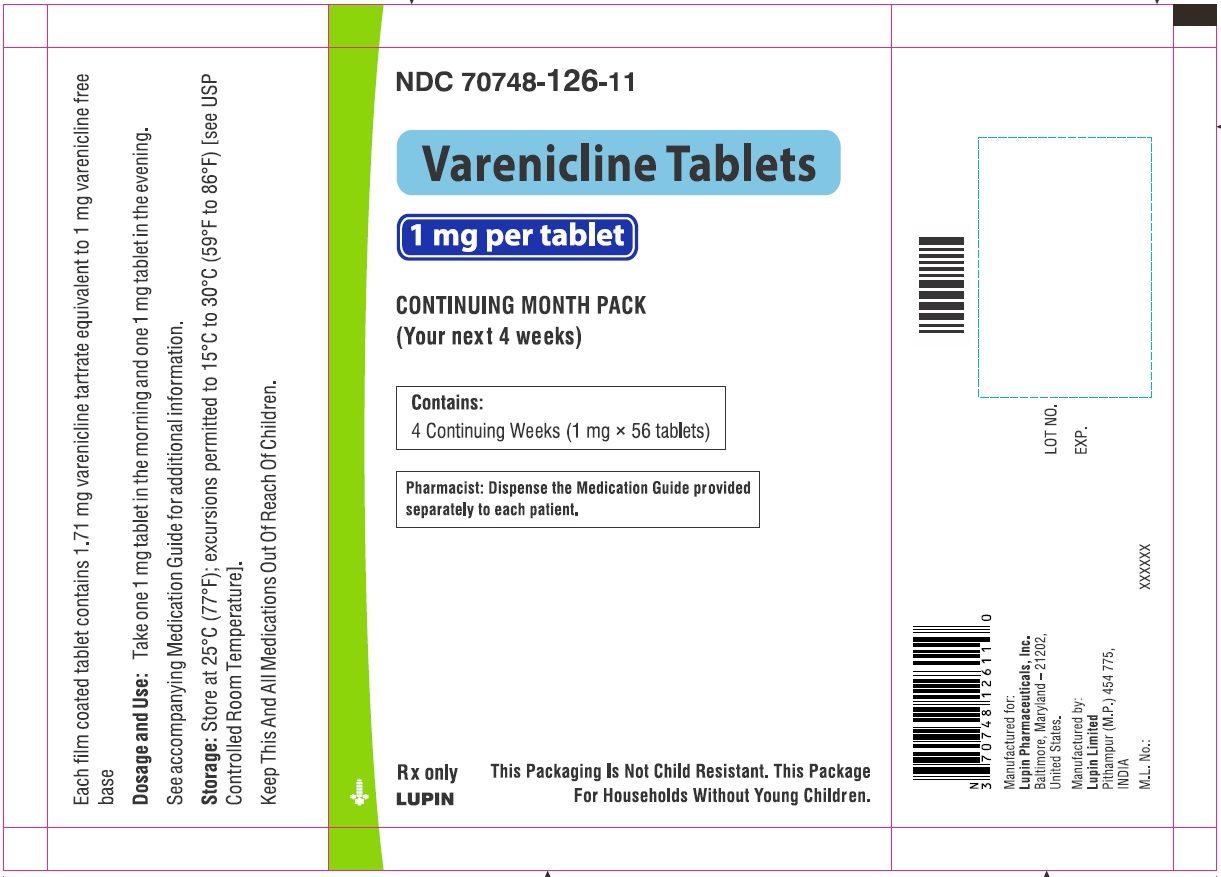
NDC 70748-125-13
Starting Month Box-Carton (with Pouch) Label
Rx only

NDC 70748-125-13
Starting Month Box-Carton (without Pouch) Label
Rx only

NDC 70748-126-13
Continuing Month Box-Carton (with Pouch) Label
Rx only
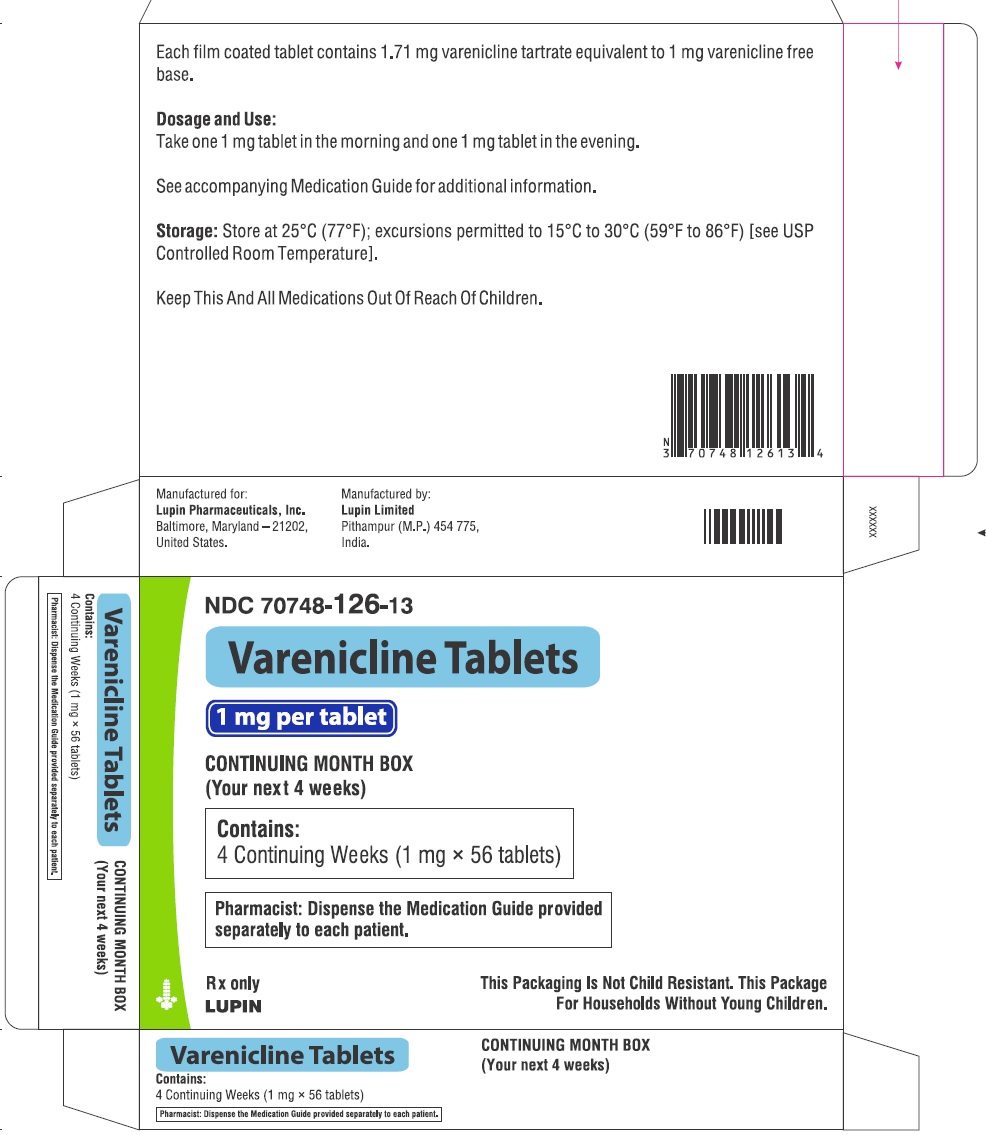
NDC 70748-126-13
Continuing Month Box-Carton (without Pouch) Label
Rx only

-
INGREDIENTS AND APPEARANCE
VARENICLINE
varenicline tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:70748-126 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength VARENICLINE TARTRATE (UNII: 82269ASB48) (VARENICLINE - UNII:W6HS99O8ZO) VARENICLINE 1 mg Inactive Ingredients Ingredient Name Strength ANHYDROUS DIBASIC CALCIUM PHOSPHATE (UNII: L11K75P92J) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) HYPROMELLOSE 2910 (3 MPA.S) (UNII: 0VUT3PMY82) MAGNESIUM STEARATE (UNII: 70097M6I30) MALTODEXTRIN (UNII: 7CVR7L4A2D) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color YELLOW Score no score Shape ROUND (BICONVEX) Size 7mm Flavor Imprint Code T;1 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70748-126-13 1 in 1 CARTON 02/16/2024 1 56 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA211862 02/16/2024 VARENICLINE TARTRATE
varenicline tartrate kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:70748-125 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70748-125-13 1 in 1 CARTON 03/20/2024 1 1 in 1 BLISTER PACK; Type 0: Not a Combination Product Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 11 Part 2 42 Part 1 of 2 VARENICLINE
varenicline tablet, film coatedProduct Information Item Code (Source) NDC:70748-368 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength VARENICLINE TARTRATE (UNII: 82269ASB48) (VARENICLINE - UNII:W6HS99O8ZO) VARENICLINE 0.5 mg Inactive Ingredients Ingredient Name Strength ANHYDROUS DIBASIC CALCIUM PHOSPHATE (UNII: L11K75P92J) MALTODEXTRIN (UNII: 7CVR7L4A2D) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) HYPROMELLOSE 2910 (3 MPA.S) (UNII: 0VUT3PMY82) MAGNESIUM STEARATE (UNII: 70097M6I30) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color WHITE (White to off white) Score no score Shape ROUND (BICONVEX) Size 8mm Flavor Imprint Code T;2 Contains Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA211862 02/16/2024 Part 2 of 2 VARENICLINE
varenicline tablet, film coatedProduct Information Item Code (Source) NDC:70748-369 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength VARENICLINE TARTRATE (UNII: 82269ASB48) (VARENICLINE - UNII:W6HS99O8ZO) VARENICLINE 1 mg Inactive Ingredients Ingredient Name Strength ANHYDROUS DIBASIC CALCIUM PHOSPHATE (UNII: L11K75P92J) MALTODEXTRIN (UNII: 7CVR7L4A2D) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) HYPROMELLOSE 2910 (3 MPA.S) (UNII: 0VUT3PMY82) MAGNESIUM STEARATE (UNII: 70097M6I30) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) TALC (UNII: 7SEV7J4R1U) Product Characteristics Color YELLOW Score no score Shape ROUND (BICONVEX) Size 7mm Flavor Imprint Code T;1 Contains Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA211862 02/16/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA211862 03/20/2024 VARENICLINE
varenicline tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:70748-127 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength VARENICLINE TARTRATE (UNII: 82269ASB48) (VARENICLINE - UNII:W6HS99O8ZO) VARENICLINE 0.5 mg Inactive Ingredients Ingredient Name Strength ANHYDROUS DIBASIC CALCIUM PHOSPHATE (UNII: L11K75P92J) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) HYPROMELLOSE 2910 (3 MPA.S) (UNII: 0VUT3PMY82) MAGNESIUM STEARATE (UNII: 70097M6I30) MALTODEXTRIN (UNII: 7CVR7L4A2D) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color WHITE (White to Off White) Score no score Shape ROUND (BICONVEX) Size 6mm Flavor Imprint Code T;2 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70748-127-49 56 in 1 BOTTLE; Type 0: Not a Combination Product 01/09/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA211862 01/09/2024 VARENICLINE
varenicline tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:70748-128 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength VARENICLINE TARTRATE (UNII: 82269ASB48) (VARENICLINE - UNII:W6HS99O8ZO) VARENICLINE 1 mg Inactive Ingredients Ingredient Name Strength ANHYDROUS DIBASIC CALCIUM PHOSPHATE (UNII: L11K75P92J) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) HYPROMELLOSE 2910 (3 MPA.S) (UNII: 0VUT3PMY82) MAGNESIUM STEARATE (UNII: 70097M6I30) MALTODEXTRIN (UNII: 7CVR7L4A2D) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color YELLOW Score no score Shape ROUND (BICONVEX) Size 7mm Flavor Imprint Code T;1 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70748-128-49 56 in 1 BOTTLE; Type 0: Not a Combination Product 01/09/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA211862 01/09/2024 Labeler - Lupin Pharmaceuticals, Inc. (089153071) Registrant - LUPIN LIMITED (675923163) Establishment Name Address ID/FEI Business Operations LUPIN LIMITED 650582310 MANUFACTURE(70748-125, 70748-126, 70748-127, 70748-128) , PACK(70748-125, 70748-126, 70748-127, 70748-128, 70748-368, 70748-369)