Label: RIVASTIGMINE patch, extended release
-
NDC Code(s):
70710-1196-1,
70710-1196-7,
70710-1197-1,
70710-1197-7, view more70710-1198-1, 70710-1198-7
- Packager: Zydus Pharmaceuticals USA Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated May 27, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use RIVASTIGMINE TRANSDERMAL SYSTEM safely and effectively. See full prescribing information for RIVASTIGMINE TRANSDERMAL ...These highlights do not include all the information needed to use RIVASTIGMINE TRANSDERMAL SYSTEM safely and effectively. See full prescribing information for RIVASTIGMINE TRANSDERMAL SYSTEM.
Rivastigmine Transdermal System
Initial U.S. Approval: 2000INDICATIONS AND USAGE
DOSAGE AND ADMINISTRATION
- Apply transdermal system on intact skin for a 24-hour period; replace with a new transdermal system every 24 hours. (2.1, 2.4)
- Initial Dose: Initiate treatment with 4.6 mg/24 hours rivastigmine transdermal system. (2.1)
- Dose Titration:
After a minimum of 4 weeks, if tolerated, increase dose to 9.5 mg/24 hours, which is the minimum effective dose. Following a minimum additional 4 weeks, may increase dosage to maximum dosage of 13.3 mg/24 hours. (2.1)
- Mild-to-Moderate Alzheimer's Disease and Parkinson's Disease Dementia : Rivastigmine transdermal system 9.5 mg/24 hours or 13.3 mg/24 hours once daily. (2.1)
- Severe Alzheimer's Disease : Rivastigmine transdermal system 13.3 mg/24 hours once daily. (2.1)
- For treatment interruption longer than 3 days, retitrate dosage starting at 4.6 mg per 24 hours. (2.1)
- Consider dose adjustments in patients with (2.2):
○ Mild-to-moderate hepatic impairment (8.6)
○ Low (less than 50 kg) body weight (8.7)
DOSAGE FORMS AND STRENGTHS
Rivastigmine Transdermal System: 4.6 mg/24 hours or 9.5 mg/24 hours or 13.3 mg/24 hours (3)
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- Hospitalization and, rarely, death have been reported due to application of multiple transdermal systems at same time. Ensure patients or caregivers receive instruction on proper dosing and administration. (5.1)
- Gastrointestinal Adverse Reactions: May include significant nausea, vomiting, diarrhea, anorexia/decreased appetite, and weight loss, and may necessitate treatment interruption. Dehydration may result from prolonged vomiting or diarrhea and can be associated with serious outcomes. (5.2)
- Application-site reactions may occur with the transdermal system form of rivastigmine. Discontinue treatment if application-site reactions spread beyond the transdermal system size, if there is evidence of a more intense local reaction (e.g., increasing erythema, edema, papules, vesicles), and if symptoms do not significantly improve within 48 hours after transdermal system removal. (5.3)
ADVERSE REACTIONS
Most common adverse reactions (less than 5% and higher than with placebo): Nausea, vomiting, and diarrhea. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Zydus Pharmaceuticals (USA) Inc. at 1-877-993-8779 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 5/2024
Close -
Table of ContentsTable of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Alzheimer’s Disease
1.2 Parkinson’s Disease Dementia
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosing
2.2 Dosing in Specific Populations
2.3 Switching to Rivastigmine Transdermal System from Rivastigmine Capsules or Rivastigmine Oral Solution
2.4 Important Administration Instructions
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Medication Errors Resulting in Overdose
5.2 Gastrointestinal Adverse Reactions
5.3 Skin Reactions
5.4 Other Adverse Reactions From Increased Cholinergic Activity
5.5 Impairment in Driving or Use of Machinery
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Metoclopramide
7.2 Cholinomimetic and Anticholinergic Medications
7.3 Beta-Blockers
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Hepatic Impairment
8.7 Low or High Body Weight
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE1.1 Alzheimer’s Disease - Rivastigmine transdermal system is indicated for the treatment of dementia of the Alzheimer's type (AD). Efficacy has been demonstrated in patients with mild, moderate ...
1.1 Alzheimer’s Disease
Rivastigmine transdermal system is indicated for the treatment of dementia of the Alzheimer's type (AD). Efficacy has been demonstrated in patients with mild, moderate, and severe Alzheimer's disease.
Close1.2 Parkinson’s Disease Dementia
Rivastigmine transdermal system is indicated for the treatment of mild-to-moderate dementia associated with Parkinson's disease (PDD).
-
2 DOSAGE AND ADMINISTRATION2.1 Recommended Dosing - Initial Dose - Initiate treatment with one 4.6 mg/24 hours rivastigmine transdermal system applied to the skin once daily [see Dosage and Administration (2.4)]. Dose ...
2.1 Recommended Dosing
Initiate treatment with one 4.6 mg/24 hours rivastigmine transdermal system applied to the skin once daily [see Dosage and Administration (2.4)].
Dose Titration
Increase the dose only after a minimum of 4 weeks at the previous dose, and only if the previous dose has been tolerated. For mild-to-moderate AD and PDD patients, continue the effective dose of 9.5 mg/24 hours for as long as therapeutic benefit persists. Patients can then be increased to the maximum effective dose of 13.3 mg/24 hours dose. For patients with severe AD, 13.3 mg/24 hours is the effective dose. Doses higher than 13.3 mg/24 hours confer no appreciable additional benefit, and are associated with an increase in the incidence of adverse reactions [see Warnings and Precautions (5.2), Adverse Reactions (6.1)].
Mild-to-Moderate Alzheimer's Disease and Mild-to-Moderate Parkinson's Disease Dementia
The effective dosage of rivastigmine transdermal system is 9.5 mg/24 hours or 13.3 mg/24 hours administered once per day; replace with a new transdermal system every 24 hours.
Severe Alzheimer's Disease
The effective dosage of rivastigmine transdermal system in patients with severe Alzheimer's disease is 13.3 mg/24 hours administered once per day; replace with a new transdermal system every 24 hours.
Interruption of Treatment
If dosing is interrupted for 3 days or fewer, restart treatment with the same or lower strength rivastigmine transdermal system. If dosing is interrupted for more than 3 days, restart treatment with the 4.6 mg/24 hours rivastigmine transdermal system and titrate as described above.
2.2 Dosing in Specific Populations
Dosing Modifications in Patients with Hepatic Impairment
Consider using the 4.6 mg/24 hours rivastigmine transdermal system as both the initial and maintenance dose in patients with mild (Child-Pugh score 5 to 6) to moderate (Child-Pugh score 7 to 9) hepatic impairment [see Use in Specific Populations (8.6), Clinical Pharmacology (12.3)].
Dosing Modifications in Patients with Low Body Weight
Carefully titrate and monitor patients with low body weight (less than 50 kg) for toxicities (e.g., excessive nausea, vomiting), and consider reducing the maintenance dose to the 4.6 mg/24 hours rivastigmine transdermal system if such toxicities develop.
2.3 Switching to Rivastigmine Transdermal System from Rivastigmine Capsules or Rivastigmine Oral Solution
Patients treated with rivastigmine capsules or oral solution may be switched to rivastigmine transdermal system as follows:
- A patient who is on a total daily dose of less than 6 mg of oral rivastigmine can be switched to the 4.6 mg/24 hours rivastigmine transdermal system.
- A patient who is on a total daily dose of 6 mg to 12 mg of oral rivastigmine can be switched to the 9.5 mg/24 hours rivastigmine transdermal system.
Instruct patients or caregivers to apply the first transdermal system on the day following the last oral dose.
Close2.4 Important Administration Instructions
Rivastigmine transdermal system is for transdermal use on intact skin.
a) Do not use the transdermal system if the pouch seal is broken or the transdermal system is cut, damaged, or changed in any way.
b) Apply the rivastigmine transdermal system once a day.
- Press down firmly for 30 seconds until the edges stick well when applying to clean, dry, hairless, intact healthy skin in a place that will not be rubbed against by tight clothing.
- Use the upper or lower back as the site of application because the transdermal system is less likely to be removed by the patient. If sites on the back are not accessible, apply the transdermal system to the upper arm or chest.
- Do not apply to a skin area where cream, lotion, or powder has recently been applied.
c) Do not apply to skin that is red, irritated, or cut.
d) Replace the rivastigmine transdermal system with a new transdermal system every 24 hours. Instruct patients to only wear 1 transdermal system at a time (remove the previous day's transdermal system before applying a new transdermal system) [see Warnings and Precautions (5.1), Overdosage (10)]. If a transdermal system falls off or if a dose is missed, apply a new transdermal system immediately and then replace this transdermal system the following day at the usual application time.
e) Change the site of transdermal system application daily to minimize potential irritation, although a new transdermal system can be applied to the same general anatomic site (e.g., another spot on the upper back) on consecutive days. Do not apply a new transdermal system to the same location for at least 14 days.
f) May wear the transdermal system during bathing and in hot weather. Avoid long exposure to external heat sources (excessive sunlight, saunas, solariums).
g) Place used transdermal systems in the previously saved pouch and discard in the trash, away from pets or children.
h) Wash hands with soap and water after removing the transdermal system. In case of contact with eyes or if the eyes become red after handling the transdermal system, rinse immediately with plenty of water, and seek medical advice if symptoms do not resolve.
-
3 DOSAGE FORMS AND STRENGTHSRivastigmine transdermal system is available in 3 strengths. Each transdermal system has a beige backing layer labeled as either: Rivastigmine transdermal system 4.6 mg/24 hours ...Close
Rivastigmine transdermal system is available in 3 strengths. Each transdermal system has a beige backing layer labeled as either:
- Rivastigmine transdermal system 4.6 mg/24 hours, "Rivastigmine Transdermal System 4.6 mg/24 hours"
- Rivastigmine transdermal system 9.5 mg/24 hours, "Rivastigmine Transdermal System 9.5 mg/24 hours"
- Rivastigmine transdermal system 13.3 mg/24 hours, "Rivastigmine Transdermal System 13.3 mg/24 hours"
-
4 CONTRAINDICATIONSRivastigmine transdermal system is contraindicated in patients with: known hypersensitivity to rivastigmine, other carbamate derivatives, or other components of the formulation [see ...Close
Rivastigmine transdermal system is contraindicated in patients with:
- known hypersensitivity to rivastigmine, other carbamate derivatives, or other components of the formulation [see Description (11)].
- previous history of application-site reactions with rivastigmine transdermal system suggestive of allergic contact dermatitis [see Warnings and Precautions (5.3)].
Isolated cases of generalized skin reactions have been described in postmarketing experience [see Adverse Reactions (6.2)].
-
5 WARNINGS AND PRECAUTIONS5.1 Medication Errors Resulting in Overdose - Medication errors with rivastigmine transdermal system have resulted in serious adverse reactions; some cases have required hospitalization, and ...
5.1 Medication Errors Resulting in Overdose
Medication errors with rivastigmine transdermal system have resulted in serious adverse reactions; some cases have required hospitalization, and rarely, led to death. The majority of medication errors have involved not removing the old transdermal system when putting on a new one and the use of multiple transdermal systems at one time.
Instruct patients and their caregivers on important administration instructions for rivastigmine transdermal system. [see Dosage and Administration (2.4)].
5.2 Gastrointestinal Adverse Reactions
Rivastigmine transdermal system can cause gastrointestinal adverse reactions, including significant nausea, vomiting, diarrhea, anorexia/decreased appetite, and weight loss. Dehydration may result from prolonged vomiting or diarrhea and can be associated with serious outcomes. The incidence and severity of these reactions are dose-related [see Adverse Reactions (6.1)]. For this reason, initiate treatment with rivastigmine transdermal system at a dose of 4.6 mg/24 hours and titrate to a dose of 9.5 mg/24 hours and then to a dose of 13.3 mg/24 hours, if appropriate [see Dosage and Administration (2.1)].
If treatment is interrupted for more than 3 days because of intolerance, reinitiate rivastigmine transdermal system with the 4.6 mg/24 hours dose to reduce the possibility of severe vomiting and its potentially serious sequelae. A postmarketing report described a case of severe vomiting with esophageal rupture following inappropriate reinitiation of treatment of an oral formulation of rivastigmine without retitration after 8 weeks of treatment interruption.
Inform caregivers to monitor for gastrointestinal adverse reactions and to inform the physician if they occur. It is critical to inform caregivers that if therapy has been interrupted for more than 3 days because of intolerance, the next dose should not be administered without contacting the physician regarding proper retitration.
5.3 Skin Reactions
Skin application-site reactions may occur with rivastigmine transdermal system. These reactions are not in themselves an indication of sensitization. However, use of rivastigmine transdermal system may lead to allergic contact dermatitis.
Allergic contact dermatitis should be suspected if application-site reactions spread beyond the transdermal system size, if there is evidence of a more intense local reaction (e.g., increasing erythema, edema, papules, vesicles) and if symptoms do not significantly improve within 48 hours after transdermal system removal. In these cases, treatment should be discontinued [see Contraindications (4)].
In patients who develop application site reactions to rivastigmine transdermal system, suggestive of allergic contact dermatitis and who still require rivastigmine, treatment should be switched to oral rivastigmine only after negative allergy testing and under close medical supervision. It is possible that some patients sensitized to rivastigmine by exposure to rivastigmine transdermal system may not be able to take rivastigmine in any form.
There have been isolated postmarketing reports of patients experiencing disseminated allergic dermatitis when administered rivastigmine irrespective of the route of administration (oral or transdermal). In these cases, treatment should be discontinued [see Contraindications (4)]. Patients and caregivers should be instructed accordingly.
5.4 Other Adverse Reactions From Increased Cholinergic Activity
Extrapyramidal Symptoms: Cholinomimetics, including rivastigmine, may exacerbate or induce extrapyramidal symptoms. Worsening of parkinsonian symptoms, particularly tremor, has been observed in patients with dementia associated with Parkinson's disease who were treated with rivastigmine capsules.
Seizures: Drugs that increase cholinergic activity are believed to have some potential for causing seizures. However, seizure activity also may be a manifestation of Alzheimer's disease.
Peptic Ulcers/Gastrointestinal Bleeding
Cholinesterase inhibitors, including rivastigmine, may increase gastric acid secretion due to increased cholinergic activity. Monitor patients using rivastigmine transdermal system for symptoms of active or occult gastrointestinal bleeding, especially those at increased risk for developing ulcers, e.g., those with a history of ulcer disease or those receiving concurrent nonsteroidal anti-inflammatory drugs (NSAIDs). Clinical studies of rivastigmine have shown no significant increase, relative to placebo, in the incidence of either peptic ulcer disease or gastrointestinal bleeding.
Use with Anesthesia
Rivastigmine, as a cholinesterase inhibitor, is likely to exaggerate succinylcholine-type muscle relaxation during anesthesia.
Cardiac Conduction Effects
Because rivastigmine increases cholinergic activity, use of the rivastigmine transdermal system may have vagotonic effects on heart rate (e.g., bradycardia). The potential for this action may be particularly important in patients with sick sinus syndrome or other supraventricular cardiac conduction conditions.
Genitourinary Effects
Although not observed in clinical trials of rivastigmine, drugs that increase cholinergic activity may cause urinary obstruction.
Pulmonary Effects
Drugs that increase cholinergic activity, including rivastigmine transdermal system should be used with care in patients with a history of asthma or obstructive pulmonary disease.
Close5.5 Impairment in Driving or Use of Machinery
Dementia may cause gradual impairment of driving performance or compromise the ability to use machinery. The administration of rivastigmine may also result in adverse reactions that are detrimental to these functions. During treatment with rivastigmine transdermal system, routinely evaluate the patient's ability to continue driving or operating machinery.
-
6 ADVERSE REACTIONSThe following clinically significant adverse reactions are described below and elsewhere in the labeling: Gastrointestinal Adverse Reactions [see Warnings and Precautions (5.2)] Skin ...
The following clinically significant adverse reactions are described below and elsewhere in the labeling:
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Rivastigmine transdermal system has been administered to 4516 patients with Alzheimer's disease during clinical trials worldwide. Of these, 3005 patients have been treated for at least 26 weeks, 1771 patients have been treated for at least 52 weeks, 974 patients have been treated for at least 78 weeks, and 24 patients have been treated for at least 104 weeks.
Mild-to-Moderate Alzheimer's Disease
24-Week International Placebo-Controlled Trial (Study 1)
Most Common Adverse Reactions
The most common adverse reactions in patients administered rivastigmine transdermal system in Study 1 [see Clinical Studies (14)], defined as those occurring at a frequency of at least 5% in the 9.5 mg/24 hours rivastigmine transdermal system arm and at a frequency at higher than in the placebo group, were nausea, vomiting, and diarrhea. These reactions were dose-related, with each being more common in patients using the unapproved 17.4 mg/24 hours rivastigmine transdermal system than in those using the 9.5 mg/24 hours rivastigmine transdermal system.
Discontinuation Rates
In Study 1, which randomized a total of 1195 patients, the proportions of patients in the rivastigmine transdermal system 9.5 mg/24 hours, rivastigmine capsules 6 mg twice daily, and placebo groups who discontinued treatment due to adverse events were 10%, 8% and 5%, respectively.
The most common adverse reactions in the rivastigmine transdermal system-treated groups that led to treatment discontinuation in this study were nausea and vomiting. The proportions of patients who discontinued treatment due to nausea were 0.7%, 1.7%, and 1.3% in the rivastigmine transdermal system 9.5 mg/24 hours, rivastigmine capsules 6 mg twice daily, and placebo groups, respectively. The proportions of patients who discontinued treatment due to vomiting were 0%, 2.0%, and 0.3% in the rivastigmine transdermal system 9.5 mg/24 hours, rivastigmine capsules 6 mg twice daily, and placebo groups, respectively.
Adverse Reactions Observed at an Incidence of Greater than or Equal to 2%
Table 1 lists adverse reactions seen at an incidence of greater than or equal to 2% in either rivastigmine transdermal system-treated group in Study 1 and for which the rate of occurrence was greater for patients treated with that dose of rivastigmine transdermal system than for those treated with placebo. The unapproved 17.4 mg/24 hours rivastigmine transdermal system arm is included to demonstrate the increased rates of gastrointestinal adverse reactions over those seen with the 9.5 mg/24 hours rivastigmine transdermal system.
Table 1
Proportion of Adverse Reactions Observed With a Frequency of Greater Than or Equal to 2% and Occurring at a Rate Greater Than Placebo in Study 1
Abbreviation: ARs, adverse reactions.
Adverse reaction
Rivastigmine Transdermal System 9.5 mg/24 hours
Rivastigmine Transdermal System 17.4 mg/24 hours
Rivastigmine Capsule 6 mg twice daily
Placebo
Total patients studied
291
303
294
302
Total percentage of patients with ARs (%)
51
66
63
46
Nausea
7
21
23
5
Vomiting*
6
19
17
3
Diarrhea
6
10
5
3
Depression
4
4
4
1
Headache
3
4
6
2
Anxiety
3
3
2
1
Anorexia/Decreased appetite
3
9
9
2
Weight Decreased **
3
8
5
1
Dizziness
2
7
7
2
Abdominal pain
2
4
1
1
Urinary tract infection
2
2
1
1
Asthenia
2
3
6
1
Fatigue
2
2
1
1
Insomnia
1
4
2
2
Abdominal pain upper
1
3
2
2
Vertigo
0
2
1
1
*Vomiting was severe in 0% of patients who received rivastigmine transdermal system 9.5 mg/24 hours, 1% of patients who received rivastigmine transdermal system 17.4 mg/24 hours, 1% of patients who received the rivastigmine capsule at doses up to 6 mg twice daily, and 0% of those who received placebo.
**Weight Decreased as presented in Table 1 is based upon clinical observations and/or adverse events reported by patients or caregivers. Body weight was also monitored at prespecified time points throughout the course of the clinical study. The proportion of patients who had weight loss equal to or greater than 7% of their baseline weight was 8% of those treated with rivastigmine transdermal system 9.5 mg/24 hours, 12% of those treated with rivastigmine transdermal system 17.4 mg/24 hours, 11% of patients who received the rivastigmine capsule at doses up to 6 mg twice daily and 6% of those who received placebo. It is not clear how much of the weight loss was associated with anorexia, nausea, vomiting, and the diarrhea associated with the drug.
48-Week International Active Comparator-Controlled Trial (Study 2)
Most Common Adverse Reactions
In Study 2 [see Clinical Studies (14)] of the commonly observed adverse reactions (greater than or equal to 3% in any treatment group), the most frequent event in the rivastigmine transdermal system 13.3 mg/24 hours group was nausea, followed by vomiting, fall, weight decreased, application-site erythema, decreased appetite, diarrhea and urinary tract infection (Table 3). The percentage of patients with these events was higher in the rivastigmine transdermal system 13.3 mg/24 hours group than in the rivastigmine transdermal system 9.5 mg/24 hours group. Patients with nausea, vomiting, diarrhea and decreased appetite experienced these reactions more often during the first 4 weeks of the double-blind treatment phase. These reactions decreased over time in each treatment group. Weight decreased was reported to have increased over time in each treatment group.
Discontinuation Rates
Table 2 displays the most common adverse reactions leading to discontinuation during the 48-week, double-blind treatment phase in Study 2.
Table 2
Proportion of Most Common Adverse Reactions (Greater Than 1% at any Dose) Leading to Discontinuation During 48-week Double-Blind Treatment Phase in Study 2
Abbreviation: ARs, adverse reactions.
Adverse reaction
Rivastigmine Transdermal System 13.3 mg/24 hours
Rivastigmine Transdermal System
9.5 mg/24 hours
Total
Total patients studied
280
283
563
Total percentage of patients with ARs leading to discontinuation (%)
9.6
12.7
11.2
Vomiting
1.4
0.4
0.9
Application-site pruritus
1.1
1.1
1.1
Aggression
0.4
1.1
0.7
Most Common Adverse Reactions Greater than or Equal to 3%
Other adverse reactions of interest which occurred less frequently, but which were observed in a markedly higher percentage of patients in the rivastigmine transdermal system 13.3 mg/24 hours group than in the rivastigmine transdermal system 9.5 mg/24 hours group in Study 2, included dizziness and upper abdominal pain. The percentage of patients with these reactions decreased over time in each treatment group (Table 3). The adverse reaction severity profile was generally similar for both the rivastigmine transdermal system 13.3 mg/24 hours and 9.5 mg/24 hours groups.
Table 3
Proportion of Adverse Reactions Over Time in the 48-week Double-Blind Treatment Phase (at least 3% in any Treatment Group) in Study 2
Abbreviations: DB, double blind; ARs, adverse reactions.
Cumulative week 0 to 48
(DB phase)
week 0 to 24
(DB phase)
week > 24 to 48
(DB phase)
Adverse reaction
Rivastigmine Transdermal System 13.3 mg/24 hours
Rivastigmine Transdermal System 9.5 mg/24 hours
Rivastigmine Transdermal System 13.3 mg/24 hours
Rivastigmine Transdermal System 9.5 mg/24 hours
Rivastigmine Transdermal System 13.3 mg/24 hours
Rivastigmine Transdermal System 9.5 mg/24 hours
Total patients studied
280
283
280
283
241
246
Total percentage of patients with ARs (%)
75
68
65
55
42
40
Nausea
12
5
10
4
4
2
Vomiting
10
5
9
3
3
2
Fall
8
6
4
4
4
3
Weight decreased*
7
3
3
1
5
2
Application-site erythema
6
6
6
5
1
2
Decreased appetite
6
3
5
2
2
<1
Diarrhea
6
5
5
4
2
<1
Urinary tract infection
5
4
3
3
3
2
Agitation
5
5
4
3
1
2
Depression
5
5
3
3
3
2
Dizziness
4
1
3
<1
2
<1
Application-site pruritus
4
4
4
3
<1
1
Headache
4
4
4
4
<1
<1
Insomnia
4
3
2
1
3
2
Abdominal pain upper
4
1
3
1
1
<1
Anxiety
4
3
2
2
2
1
Hypertension
3
3
3
2
1
1
Urinary incontinence
3
2
2
1
1
<1
Psychomotor hyperactivity
3
3
2
3
2
1
Aggression
2
3
1
3
1
1
*Decreased Weight as presented in Table 3 is based upon clinical observations and/or adverse events reported by patients or caregivers. Body weight was monitored as a vital sign at pre-specified time points throughout the course of the clinical study. The proportion of patients who had weight loss equal to or greater than 7% of their baseline weight was 15.2% of those treated with rivastigmine transdermal system 9.5 mg/24 hours and 18.6% of those treated with rivastigmine transdermal system 13.3 mg/24 hours during the 48-week double-blind treatment period.
Severe Alzheimer's Disease
24-Week US Controlled Trial (Study 3)
Most Commonly Observed Adverse Reactions
The most common adverse reactions in patients administered rivastigmine transdermal system in the controlled clinical trial, defined as those occurring at a frequency of at least 5% in the 13.3 mg/24 hours rivastigmine transdermal system arm and at a frequency higher than in the 4.6 mg/24 hours rivastigmine transdermal system were application-site erythema, fall, insomnia, vomiting, diarrhea, weight decreased, and nausea (Table 4). Patients in the lower-dose group reported more events of agitation, urinary tract infection, and hallucinations than patients in the higher-dose group.
Discontinuation Rates
In Study 3 [see Clinical Studies (14)], the proportions of patients in the rivastigmine transdermal system 13.3 mg/24 hours (n=355) and rivastigmine transdermal system 4.6 mg/24 hours (n=359), who discontinued treatment due to adverse reactions were 21% and 14%, respectively.
The most frequent adverse reaction leading to discontinuation in the 13.3 mg/24 hours treatment group versus the 4.6 mg/24 hours treatment group was agitation (2.8% versus 2.2%), followed by vomiting (2.5% and 1.1%), nausea (1.7% and 1.1%), decreased appetite (1.7% and 0%), aggression (1.1% and 0.3%), fall (1.1% and 0.3%) and syncope (1.1% and 0.3%). Otherwise, all AEs leading to discontinuation were reported in less than 1% of patients.
Most Commonly Observed Adverse Reactions Greater than or Equal to 5%
Other adverse reactions of interest, which were observed in a higher percentage of patients in the rivastigmine transdermal system 13.3 mg/24 hours group than in the rivastigmine transdermal system 4.6 mg/24 hours group, included application-site erythema, fall, insomnia, vomiting, diarrhea, weight decreased, and nausea (Table 4). Overall, the majority of patients in this study experienced adverse reactions that were mild (30.7%) or moderate (32.1%) in severity. Slightly more patients in the 4.6 mg/24 hours transdermal system group reported mild events than in the 13.3 mg/24 hours transdermal system group, while the numbers of patients reporting moderate events were comparable between groups. Severe adverse reactions were reported at a slightly higher percentage at the higher dose (12.4%) than at the lower-dose (10%) treatment groups. With the exception of severe adverse reactions of agitation (13.3 mg: 1.1%; 4.6 mg: 1.4%), fall (13.3 mg: 1.1%) and urinary tract infection (4.6 mg: 1.1%), all adverse reactions reported as severe occurred in less than 1% of patients in either treatment group.
Table 4
Proportion of Adverse Reactions in the 24-week Double-Blind Treatment Phase (at least 5% in any Treatment Group) in Study 3
Abbreviation: ARs, adverse reactions.
Adverse reaction
Rivastigmine Transdermal System
13.3 mg/24 hours
Rivastigmine Transdermal System
4.6 mg/24 hours
Total number of patients studied
355
359
Total percentage of patients with ARs (%)
75
73
Application-site erythema
13
12
Agitation
12
14
Urinary tract infection
8
10
Fall
8
6
Insomnia
7
4
Vomiting
7
3
Diarrhea
7
5
Weight decreased*
7
3
Nausea
6
3
Depression
5
4
Decreased appetite
5
1
Anxiety
5
5
Hallucination
2
5
*Weight Decreased as presented in Table 4 is based upon clinical observations and/or adverse events reported by patients or caregivers. Body weight was monitored as a vital sign at prespecified time points throughout the course of the clinical study. The proportion of patients who had weight loss equal to or greater than 7% of their baseline weight was 11% of those treated with rivastigmine transdermal system 4.6 mg/24 hours and 14.1% of those treated with rivastigmine transdermal system 13.3 mg/24 hours during the 24-week double-blind treatment.
Application-Site Reactions
Application-site skin reactions leading to discontinuation were observed in less than or equal to 2.3% of rivastigmine transdermal system patients. This number was 4.9% and 8.4% in the Chinese population and Japanese population, respectively.
Cases of skin irritation were captured separately on an investigator-rated skin irritation scale. Skin irritation, when observed, was mostly slight or mild in severity and was rated as severe in less than or equal to 2.2% of rivastigmine transdermal system patients in a double-blind controlled study and in less than or equal to 3.7% of rivastigmine transdermal system patients in a double-blind controlled study in Japanese patients.
Parkinson's Disease Dementia
76-week International Open-Label Trial (Study 4)
Rivastigmine transdermal system has been administered to 288 patients with mild-to-moderate Parkinson's Disease Dementia in a single, 76-week, open-label, active-comparator safety study. Of these, 256 have been treated for at least 12 weeks, 232 for at least 24 weeks, and 196 for at least 52 weeks.
Treatment with rivastigmine transdermal system was initiated at 4.6 mg/24 hours and if tolerated the dose was increased after 4 weeks to 9.5 mg/24 hours. Rivastigmine capsule (target maintenance dose of 12 mg/day) served as the active comparator and was administered to 294 patients. Adverse reactions are presented in Table 5.
Table 5
Proportion of Adverse Reactions Reported at a Rate Greater Than or Equal to 2% During the Initial 24-Week Period in Study 4
Adverse Reaction
Rivastigmine Transdermal System
Total patients studied
288
Percentage (%)
Psychiatric disorders
Insomnia
6
Depression
6
Anxiety
5
Agitation
3
Nervous system disorders
Tremor
7
Dizziness
6
Somnolence
4
Hypokinesia
4
Bradykinesia
4
Cogwheel rigidity
3
Dyskinesia
3
Gastrointestinal disorders
Abdominal pain
2
Vascular disorders
Hypertension
3
General disorders and administration-site conditions
Fall
12
Application site erythema
11
Application site irritation, pruritus, rash
3;5;2
Fatigue
4
Asthenia
2
Gait disturbance
4
Additional adverse reactions observed during the 76-week prospective, open-label study in patients with dementia associated with Parkinson's disease treated with rivastigmine transdermal system: Frequent (those occurring in at least 1/100 patients): dehydration, weight decreased, aggression, hallucination visual.
In patients with dementia associated with Parkinson's disease, the following adverse drug reactions have only been observed in clinical trials with rivastigmine capsules: Frequent: nausea, vomiting, decreased appetite, restlessness, worsening of Parkinson's disease, bradycardia, diarrhea, dyspepsia, salivary hypersecretion, sweating increased; Infrequent (those occurring between 1/100 to 1/1000 patients): dystonia, atrial fibrillation, atrioventricular block.
Close6.2 Postmarketing Experience
The following adverse reactions have been identified during post approval use of rivastigmine capsules, rivastigmine oral solution, or rivastigmine transdermal system. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Cardiac Disorders: Tachycardia, QTc prolongation, torsades de pointes
Hepatobiliary Disorders: Abnormal liver function tests, hepatitis.
Nervous System Disorders: Parkinson's disease (worsening), seizure, tremor.
Psychiatric Disorders: nightmares.
Skin and Subcutaneous Tissue Disorders: Allergic dermatitis, application-site hypersensitivity, blister, disseminated allergic dermatitis, Stevens-Johnson syndrome, urticaria.
Vascular Disorders: Hypertension.
-
7 DRUG INTERACTIONS7.1 Metoclopramide - Due to the risk of additive extra-pyramidal adverse reactions, the concomitant use of metoclopramide and rivastigmine transdermal system is not recommended. 7.2 ...
7.1 Metoclopramide
Due to the risk of additive extra-pyramidal adverse reactions, the concomitant use of metoclopramide and rivastigmine transdermal system is not recommended.
7.2 Cholinomimetic and Anticholinergic Medications
Rivastigmine transdermal system may increase the cholinergic effects of other cholinomimetic medications and may also interfere with the activity of anticholinergic medications (e.g., oxybutynin, tolterodine). Concomitant use of rivastigmine transdermal system with medications having these pharmacologic effects is not recommended unless deemed clinically necessary [see Warnings and Precautions (5.5)].
Close7.3 Beta-Blockers
Additive bradycardic effects resulting in syncope may occur when rivastigmine is used concomitantly with beta-blockers, especially cardioselective beta-blockers (including atenolol). Concomitant use is not recommended when signs of bradycardia including syncope are present.
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - There are no adequate data on the developmental risks associated with the use of rivastigmine in pregnant women. In animals, no adverse effects on embryo-fetal ...
8.1 Pregnancy
There are no adequate data on the developmental risks associated with the use of rivastigmine in pregnant women. In animals, no adverse effects on embryo-fetal development were observed at oral doses 2-4 times the maximum recommended human dose (MRHD) (see Data).
The background risk of major birth defects and miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2%-4% and 15%-20%, respectively.
Data
Animal Data
Oral administration of rivastigmine to pregnant rats and rabbits throughout organogenesis produced no adverse effects on embryo-fetal development up to the highest dose tested (2.3 mg/kg/day), which is 2 and 4 times, respectively, the MRHD of 12 mg per day on a body surface area (mg/m2) basis.
8.2 Lactation
There are no data on the presence of rivastigmine in human milk, the effects on the breastfed infant, or the effects of rivastigmine on milk production. Rivastigmine and its metabolites are excreted in rat milk following oral administration of rivastigmine; levels of rivastigmine plus metabolites in rat milk are approximately 2 times that in maternal plasma.
The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for rivastigmine and any potential adverse effects on the breastfed infant from rivastigmine or from the underlying maternal condition.
8.4 Pediatric Use
Safety and effectiveness in pediatric patients have not been established. The use of rivastigmine transdermal system in pediatric patients (below 18 years of age) is not recommended.
8.5 Geriatric Use
Of the total number of patients in clinical studies of rivastigmine transdermal system, 88% were 65 years and over, while 55% were 75 years. No overall differences in safety or effectiveness were observed between these patients and younger patients, and other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
8.6 Hepatic Impairment
Increased exposure to rivastigmine was observed in patients with mild or moderate hepatic impairment with oral rivastigmine. Patients with mild or moderate hepatic impairment may be able to only tolerate lower doses [see Dosage and Administration (2.2), Clinical Pharmacology (12.3)]. No data are available on the use of rivastigmine in patients with severe hepatic impairment.
Close8.7 Low or High Body Weight
Because rivastigmine blood levels vary with weight, careful titration and monitoring should be performed in patients with low or high body weights [see Dosage and Administration (2.2), Clinical Pharmacology (12.3)].
-
10 OVERDOSAGEOverdose with rivastigmine transdermal system has been reported in the postmarketing setting [see Warnings and Precautions (5.1)]. Overdoses have occurred from application of more than one ...
Overdose with rivastigmine transdermal system has been reported in the postmarketing setting [see Warnings and Precautions (5.1)]. Overdoses have occurred from application of more than one transdermal system at one time and not removing the previous day's transdermal system before applying a new transdermal system. The symptoms reported in these overdose cases are similar to those seen in cases of overdose associated with rivastigmine oral formulations.
Because strategies for the management of overdose are continually evolving, it is advisable to contact a Poison Control Center to determine the latest recommendations for the management of an overdose of any drug. As rivastigmine has a plasma half-life of about 3.4 hours after transdermal system administration and a duration of acetylcholinesterase inhibition of about 9 hours, it is recommended that in cases of asymptomatic overdose the transdermal system should be immediately removed and no further transdermal system should be applied for the next 24 hours.
As in any case of overdose, general supportive measures should be utilized.
Overdosage with cholinesterase inhibitors can result in cholinergic crisis characterized by severe nausea, vomiting, salivation, sweating, bradycardia, hypotension, respiratory depression, and convulsions. Increasing muscle weakness is a possibility and may result in death if respiratory muscles are involved. Atypical responses in blood pressure and heart rate have been reported with other drugs that increase cholinergic activity when coadministered with quaternary anticholinergics such as glycopyrrolate. Additional symptoms associated with rivastigmine overdose are diarrhea, abdominal pain, dizziness, tremor, headache, somnolence, confusional state, hyperhidrosis, hypertension, hallucinations and malaise. Due to the short plasma elimination half-life of rivastigmine after transdermal system administration, dialysis (hemodialysis, peritoneal dialysis, or hemofiltration) would not be clinically indicated in the event of an overdose.
In overdose accompanied by severe nausea and vomiting, the use of antiemetics should be considered. A fatal outcome has rarely been reported with rivastigmine overdose.
Close -
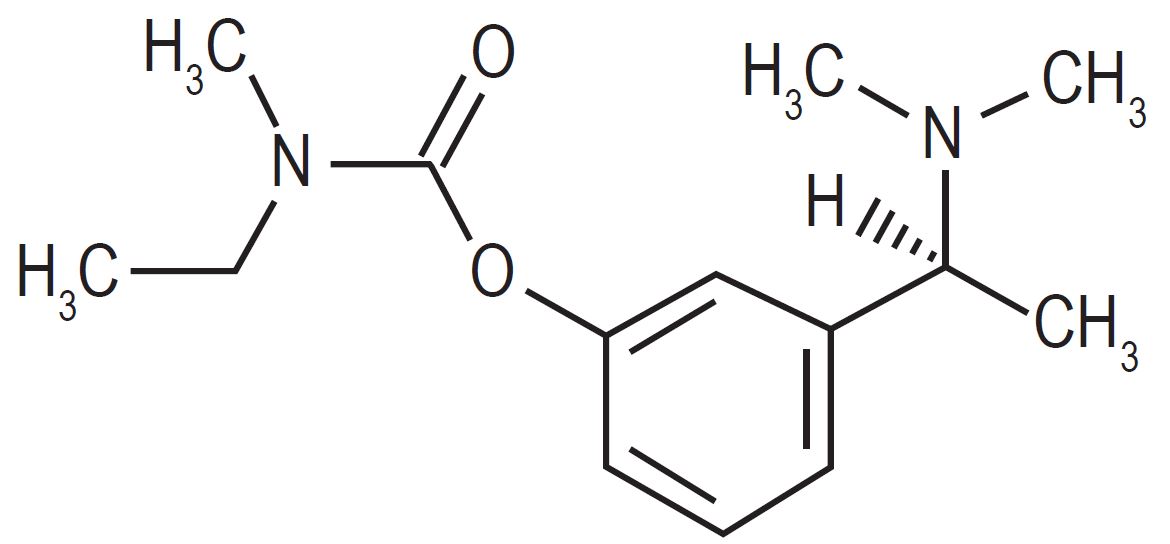
11 DESCRIPTIONRivastigmine transdermal system contains rivastigmine USP, a reversible cholinesterase inhibitor known chemically as (S)-3-[1-(dimethylamino)ethyl]phenyl ethylmethylcarbamate. It has an empirical ...
Rivastigmine transdermal system contains rivastigmine USP, a reversible cholinesterase inhibitor known chemically as (S)-3-[1-(dimethylamino)ethyl]phenyl ethylmethylcarbamate. It has an empirical formula of C14H22N2O2 as the base and a molecular weight of 250.34 g/mol (as the base). Rivastigmine is a viscous, clear, and colorless to yellow to very slightly brown liquid that is sparingly soluble in water and very soluble in ethanol, acetonitrile, n-octanol and ethyl acetate.
The distribution coefficient at 37°C in n-octanol/phosphate buffer solution pH 7 is 4.27.
Rivastigmine transdermal system is for transdermal administration. The transdermal system is a four-layer laminate containing (1) a foam backing with adhesive layer, (2) a polyester film, (3) an adhesive matrix containing rivastigmine, and (4) a fluoropolymer coated polyester release liner (see Figure 1). The release liner is removed and discarded prior to use.
Figure 1: Cross Section of the Rivastigmine Transdermal System
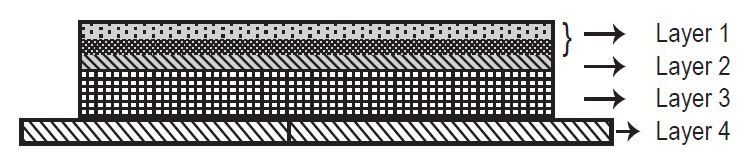
Layer 1: Copolymer foam backing with adhesive layer
Layer 2: Polyester Film
Layer 3: Adhesive Matrix
Layer 4: Fluoropolymer coated Release Liner (removed at time of use)
The active component of the system is rivastigmine. The remaining components of the system (colloidal silicon dioxide, light mineral oil, polyisobutylene adhesive, copolymer foam and polyester film) are pharmacologically inactive. Additionally, rivastigmine transdermal system is printed with pharmaceutical grade brown ink which contains acrylic polymers, carbon black, ethoxylated 2,4,7,9-tetramethyl 5 decyn-4,7-diol, iron oxide, octylphenoxypolyethoxyethanol, polyethylene glycol, polyethylene wax and polytetrafluoroethylene.
Close -
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Although the precise mechanism of action of rivastigmine is unknown, it is thought to exert its therapeutic effect by enhancing cholinergic function. This is ...
12.1 Mechanism of Action
Although the precise mechanism of action of rivastigmine is unknown, it is thought to exert its therapeutic effect by enhancing cholinergic function. This is accomplished by increasing the concentration of acetylcholine through reversible inhibition of its hydrolysis by cholinesterase. The effect of rivastigmine may lessen as the disease process advances and fewer cholinergic neurons remain functionally intact. There is no evidence that rivastigmine alters the course of the underlying dementing process.
12.2 Pharmacodynamics
After a 6-mg oral dose of rivastigmine in humans, anticholinesterase activity is present in cerebrospinal fluid for about 10 hours, with a maximum inhibition of about 60% 5 hours after dosing.
In vitro and in vivo studies demonstrate that the inhibition of cholinesterase by rivastigmine is not affected by the concomitant administration of memantine, an N-methyl-D-aspartate receptor antagonist.
Close12.3 Pharmacokinetics
After the initial application of rivastigmine transdermal system, there is a lag time of 0.5 to 1 hour in the absorption of rivastigmine. Concentrations then rise slowly typically reaching a maximum after 8 hours, although maximum values (Cmax) can also occur later (at 10 to 16 hours). After the peak, plasma concentrations slowly decrease over the remainder of the 24-hour period of application. At steady state, trough levels are approximately 60% to 80% of peak levels.
Rivastigmine transdermal system 9.5 mg/24 hours gave exposure approximately the same as that provided by an oral dose of 6 mg twice daily (i.e., 12 mg/day). Inter-subject variability in exposure was lower (43% to 49%) for the rivastigmine transdermal system formulation as compared with the oral formulations (73% to 103%). Fluctuation (between Cmax and Cmin) is less for rivastigmine transdermal system than for the oral formulation of rivastigmine.
Figure 2 displays rivastigmine plasma concentrations over 24 hours for the 3 available transdermal system strengths.
Figure 2
Rivastigmine Plasma Concentrations Following Dermal 24-Hour Transdermal System Application
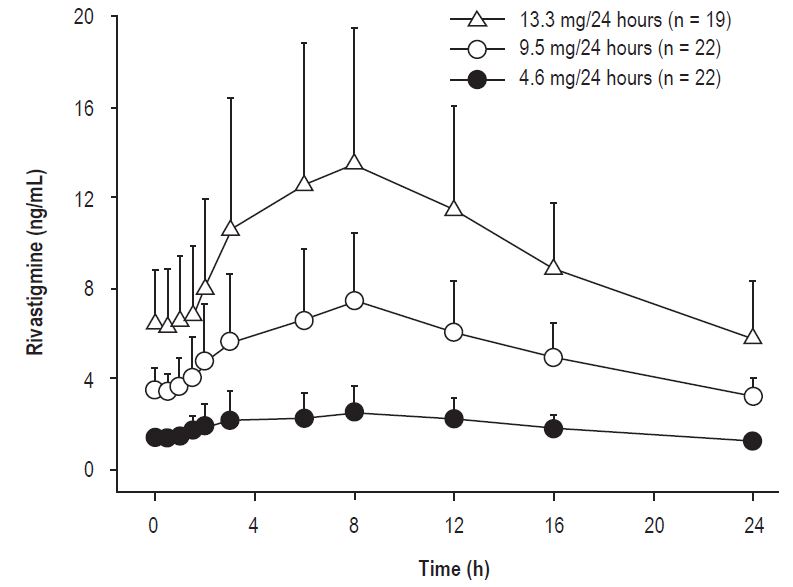
Over a 24-hour dermal application, approximately 50% of the drug content of the transdermal system is released from the system.
Exposure area under the plasma concentration-time curve from time zero to infinity (AUC∞) to rivastigmine (and metabolite NAP226-90) was highest when the transdermal system was applied to the upper back, chest, or upper arm. Two other sites (abdomen and thigh) could be used if none of the 3 other sites is available, but the practitioner should be aware that the rivastigmine plasma exposure associated with these sites was approximately 20% to 30% lower.
There was no relevant accumulation of rivastigmine or the metabolite NAP226-90 in plasma in patients with Alzheimer's disease with daily dosing.
The pharmacokinetic profile of rivastigmine transdermal systems was comparable in patients with Alzheimer's disease and in patients with dementia associated with Parkinson's disease.
Distribution
Rivastigmine is weakly bound to plasma proteins (approximately 40%) over the therapeutic range. It readily crosses the blood-brain barrier, reaching CSF peak concentrations in 1.4 to 2.6 hours. It has an apparent volume of distribution in the range of 1.8 to 2.7 L/kg.
Metabolism
Rivastigmine is extensively metabolized primarily via cholinesterase-mediated hydrolysis to the decarbamylated metabolite NAP226-90. In vitro, this metabolite shows minimal inhibition of acetylcholinesterase (less than 10%). Based on evidence from in vitro and animal studies, the major cytochrome P450 isoenzymes are minimally involved in rivastigmine metabolism.
The metabolite-to-parent (AUC∞) ratio was about 0.7 after rivastigmine transdermal system application versus 3.5 after oral administration, indicating that much less metabolism occurred after dermal treatment. Less NAP226-90 is formed following transdermal system application, presumably because of the lack of presystemic (hepatic first pass) metabolism. Based on in vitro studies, no unique metabolic routes were detected in human skin.
Elimination
Renal excretion of the metabolites is the major route of elimination. Unchanged rivastigmine is found in trace amounts in the urine. Following administration of 14C-rivastigmine, renal elimination was rapid and essentially complete (greater than 90%) within 24 hours. Less than 1% of the administered dose is excreted in the feces. The apparent elimination half-life in plasma is approximately 3 hours after transdermal system removal. Renal clearance was approximately 2.1 to 2.8 L/hr.
Age
Age had no impact on the exposure to rivastigmine in Alzheimer's disease patients treated with rivastigmine transdermal system.
Gender and Race
No specific pharmacokinetic study was conducted to investigate the effect of gender and race on the disposition of rivastigmine transdermal system. A population pharmacokinetic analysis of oral rivastigmine indicated that neither gender (n=277 males and 348 females) nor race (n=575 Caucasian, 34 black, 4 Asian, and 12 Other) affected clearance of the drug.
Similar results were seen with analyses of pharmacokinetic data obtained after the administration of rivastigmine transdermal system.
Body Weight
A relationship between drug exposure at steady state (rivastigmine and metabolite NAP226-90) and body weight was observed in Alzheimer's dementia patients. Rivastigmine exposure is higher in subjects with low body weight. Compared to a patient with a body weight of 65 kg, the rivastigmine steady-state concentrations in a patient with a body weight of 35 kg would be approximately doubled, while for a patient with a body weight of 100 kg the concentrations would be approximately halved [see Dosage and Administration (2.2)].
Renal Impairment
No study was conducted with rivastigmine transdermal system in subjects with renal impairment. Based on population analysis, creatinine clearance did not show any clear effect on steady-state concentrations of rivastigmine or its metabolite.
Hepatic Impairment
No pharmacokinetic study was conducted with rivastigmine transdermal system in subjects with hepatic impairment. Following a single 3-mg dose, mean oral clearance of rivastigmine was 60% lower in hepatically impaired patients (n=10, biopsy proven) than in healthy subjects (n=10). After multiple 6-mg twice a day oral dosing, the mean clearance of rivastigmine was 65% lower in mild (n=7, Child-Pugh score 5 to 6) and moderate (n=3, Child-Pugh score 7 to 9) hepatically impaired patients (biopsy proven, liver cirrhosis) than in healthy subjects (n=10) [see Dosage and Administration (2.2), Use in Specific Populations (8.6)].
Smoking
Following oral rivastigmine administration (up to 12 mg/day) with nicotine use, population pharmacokinetic analysis showed increased oral clearance of rivastigmine by 23% (n=75 smokers and 549 nonsmokers).
Drug Interaction Studies
No specific interaction studies have been conducted with rivastigmine transdermal system. Information presented below is from studies with oral rivastigmine.
Effect of Rivastigmine on the Metabolism of Other Drugs
Rivastigmine is primarily metabolized through hydrolysis by esterases. Minimal metabolism occurs via the major cytochrome P450 isoenzymes. Based on in vitro studies, no pharmacokinetic drug interactions with drugs metabolized by the following isoenzyme systems are expected: CYP1A2, CYP2D6, CYP3A4/5, CYP2E1, CYP2C9, CYP2C8, CYP2C19, or CYP2B6.
No pharmacokinetic interaction was observed between rivastigmine taken orally and digoxin, warfarin, diazepam, or fluoxetine in studies in healthy volunteers. The increase in prothrombin time induced by warfarin is not affected by administration of rivastigmine.
Effect of Other Drugs on the Metabolism of Rivastigmine
Drugs that induce or inhibit CYP450 metabolism are not expected to alter the metabolism of rivastigmine.
Population pharmacokinetic analysis with a database of 625 patients showed that the pharmacokinetics of rivastigmine taken orally were not influenced by commonly prescribed medications such as antacids (n=77), antihypertensives (n=72), beta-blockers (n=42), calcium channel blockers (n=75), antidiabetics (n=21), nonsteroidal anti-inflammatory drugs (n=79), estrogens (n=70), salicylate analgesics (n=177), antianginals (n=35), and antihistamines (n=15).
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - In oral carcinogenicity studies conducted at doses up to 1.1 mg/kg/day in rats and 1.6 mg/kg/day in mice, rivastigmine ...Close
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
In oral carcinogenicity studies conducted at doses up to 1.1 mg/kg/day in rats and 1.6 mg/kg/day in mice, rivastigmine was not carcinogenic.
In a dermal carcinogenicity study conducted at doses up to 0.75 mg base/kg/day in mice, rivastigmine was not carcinogenic. The mean rivastigmine plasma exposure (AUC) at this dose was less than that in humans at the maximum recommended human dose (13.3 mg/24 hours).
Mutagenesis
Rivastigmine was clastogenic in in vitro chromosomal aberration assays in mammalian cells in the presence, but not the absence, of metabolic activation. Rivastigmine was negative in an in vitro bacterial reverse mutation (Ames) assay, an in vitro HGPRT assay, and in an in vivo mouse micronucleus test.
Impairment of Fertility
No fertility or reproduction studies of dermal rivastigmine have been conducted in animals. Rivastigmine had no effect on fertility or reproductive performance in rats at oral doses up to 1.1 mg/kg/day.
-
14 CLINICAL STUDIESThe effectiveness of the rivastigmine transdermal system in dementia of the Alzheimer's type and dementia associated with Parkinson's disease was based on the results of 3 controlled trials of ...
The effectiveness of the rivastigmine transdermal system in dementia of the Alzheimer's type and dementia associated with Parkinson's disease was based on the results of 3 controlled trials of rivastigmine transdermal system in patients with Alzheimer's disease (Studies 1, 2, and 3) (see below); 3 controlled trials of oral rivastigmine in patients with dementia of the Alzheimer's type; and 1 controlled trial of oral rivastigmine in patients with dementia associated with Parkinson's disease. See the prescribing information for oral rivastigmine for details of the four studies of oral rivastigmine.
Mild-to-Moderate Alzheimer's Disease
International 24-Week Study of Rivastigmine Transdermal System in Dementia of the Alzheimer's Type (Study 1)
This study was a randomized double-blind, double dummy clinical investigation in patients with Alzheimer's disease [diagnosed by NINCDS-ADRDA and DSM-IV criteria, Mini-Mental
Status Examination (MMSE) score greater than or equal to 10 and less than or equal to 20] (Study 1). The mean age of patients participating in this trial was 74 years with a range of 50 to 90 years. Approximately 67% of patients were women, and 33% were men. The racial distribution was Caucasian 75%, black 1%, Asian 9%, and other races 15%.
The effectiveness of the rivastigmine transdermal system was evaluated in Study 1 using a dual outcome assessment strategy, evaluating for changes in both cognitive performance and overall clinical effect.
The ability of the rivastigmine transdermal system to improve cognitive performance was assessed with the cognitive subscale of the Alzheimer's-Disease Assessment Scale (ADASCog),a multi-item instrument that has been extensively validated in longitudinal cohorts of Alzheimer's- disease patients. The ADAS-Cog examines selected aspects of cognitive performance including elements of memory, orientation, attention, reasoning, language, and praxis. The ADAS-Cog scoring range is from 0 to 70, with higher scores indicating greater cognitive impairment. Elderly normal adults may score as low as 0 or 1, but it is not unusual for non-demented adults to score slightly higher.
The ability of the rivastigmine transdermal system to produce an overall clinical effect was assessed using the Alzheimer's-Disease Cooperative Study-Clinical Global Impression of Change (ADCS-CGIC). The ADCS-CGIC is a more standardized form of the Clinician's Interview-Based Impression of Change-Plus (CIBIC-Plus) and is also scored as a 7-point categorical rating; scores range from 1, indicating "markedly improved," to 4, indicating "no change," to 7, indicating "marked worsening."
In Study 1, 1195 patients were randomized to 1 of the following 4 treatments: rivastigmine transdermal system 9.5 mg/24 hours, rivastigmine transdermal system 17.4 mg/24 hours, rivastigmine capsules in a dose of 6 mg twice daily, or placebo. This 24-week study was divided into a 16-week titration phase followed by an 8-week maintenance phase. In the active treatment arms of this study, doses below the target dose were permitted during the maintenance phase in the event of poor tolerability.
Figure 3 illustrates the time course for the change from baseline in ADAS-Cog scores for all 4 treatment groups over the 24-week study. At 24 weeks, the mean differences in the ADAS-Cog change scores for the rivastigmine-treated patients compared to the patients on placebo, were 1.8, 2.9, and 1.8 units for the rivastigmine transdermal system 9.5 mg/24 hours, rivastigmine transdermal system 17.4 mg/24 hours, and rivastigmine capsule 6 mg twice daily groups, respectively. The difference between each of these groups and placebo was statistically significant. Although a slight improvement was observed with the 17.4 mg/24 hours transdermal system compared to the 9.5 mg/24 hours transdermal system on this outcome measure, no meaningful difference between the two was seen on the global evaluation (see Figure 4).
Figure 3
Time Course of the Change from Baseline in ADAS-Cog Score for Patients Observed at Each Time Point in Study 1
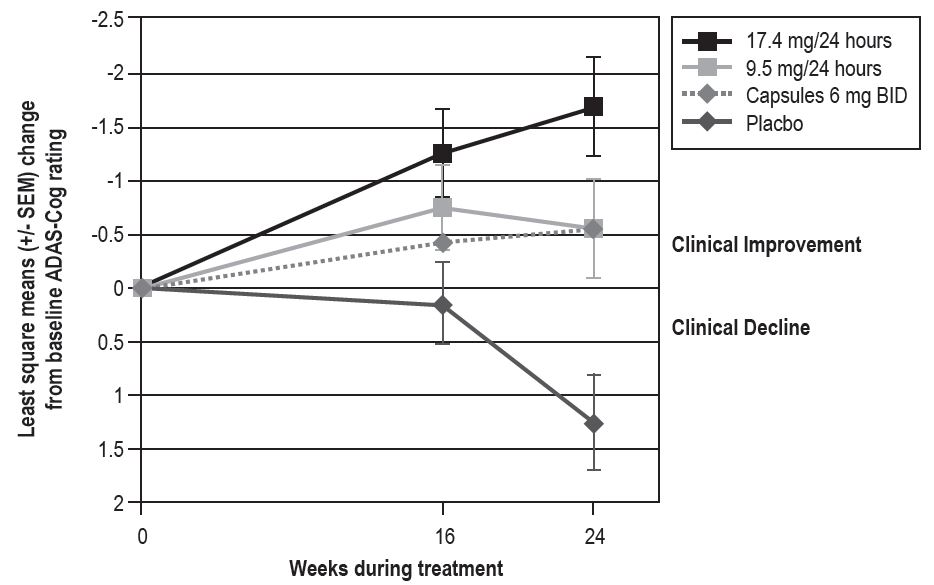
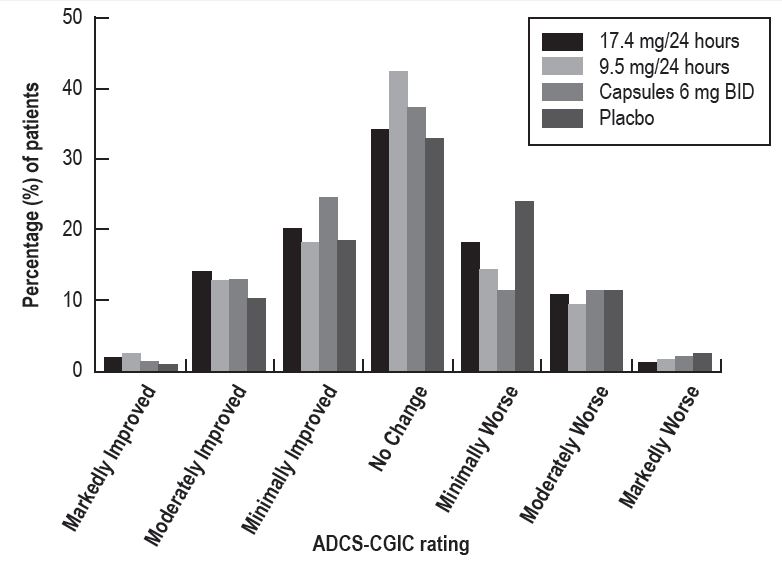
Figure 4 presents the distribution of patients' scores on the ADCS-CGIC for all 4 treatment groups. At 24 weeks, the mean difference in the ADCS-CGIC scores for the comparison of patients in each of the rivastigmine-treated groups with the patients on placebo was 0.2 units. The difference between each of these groups and placebo was statistically significant.
Figure 4
Distribution of ADCS-CGIC Scores for Patients Completing Study 1
International 48-Week Study of Rivastigmine Transdermal System in Dementia of the Alzheimer's Type (Study 2)
This study was a randomized double-blind clinical investigation in patients with Alzheimer's disease [diagnosed by NINCDS-ADRDA and DSM-IV criteria, Mini-Mental State Examination (MMSE) score greater than or equal to 10 and less than or equal to 24] (Study 2). The mean age of patients participating in this trial was 76 years with a range of 50 to 85 years. Approximately 65% of patients were women and 35% were men. The racial distribution was approximately Caucasian 97%, Black 2%, Asian 0.5%, and other races 1%. Approximately 27% of the patients were taking memantine throughout the entire duration of the study.
Alzheimer's disease patients who received 24 to 48 weeks open-label treatment with rivastigmine transdermal system 9.5 mg/24 hours and who demonstrated functional and cognitive decline were randomized into treatment with either rivastigmine transdermal system 9.5 mg/24 hours or rivastigmine transdermal system 13.3 mg/24 hours in a 48-week, double-blind treatment phase. Functional decline was assessed by the investigator and cognitive decline was defined as a decrease in the MMSE score of greater than or equal to 2 points from the previous visit or a decrease of greater than or equal to 3 points from baseline.
Study 2 was designed to compare the efficacy of rivastigmine transdermal system 13.3 mg/ 24 hours versus that of rivastigmine transdermal system 9.5 mg/24 hours during the 48-week, double-blind treatment phase.
The ability of the rivastigmine transdermal system 13.3 mg/24 hours to improve cognitive performance over that provided by the rivastigmine transdermal system 9.5 mg/24 hours was assessed by the cognitive subscale of the Alzheimer's Disease Assessment Scale (ADAS-Cog) [see Clinical Studies (14)].
The ability of the rivastigmine transdermal system 13.3 mg/24 hours to improve overall function versus that provided by rivastigmine transdermal system 9.5 mg/24 hours was assessed by the instrumental subscale of the Alzheimer's Disease Cooperative Study Activities of Daily Living (ADCS-IADL). The ADCS-IADL subscale is composed of items 7 to 23 of the caregiver-based ADCS-ADL scale. The ADCS-IADL assesses activities such as those necessary for communicating and interacting with other people, maintaining a household, and conducting hobbies and interests. A sum score is calculated by adding the scores of the individual items and can range from 0 to 56, with higher scores indicating less impairment.
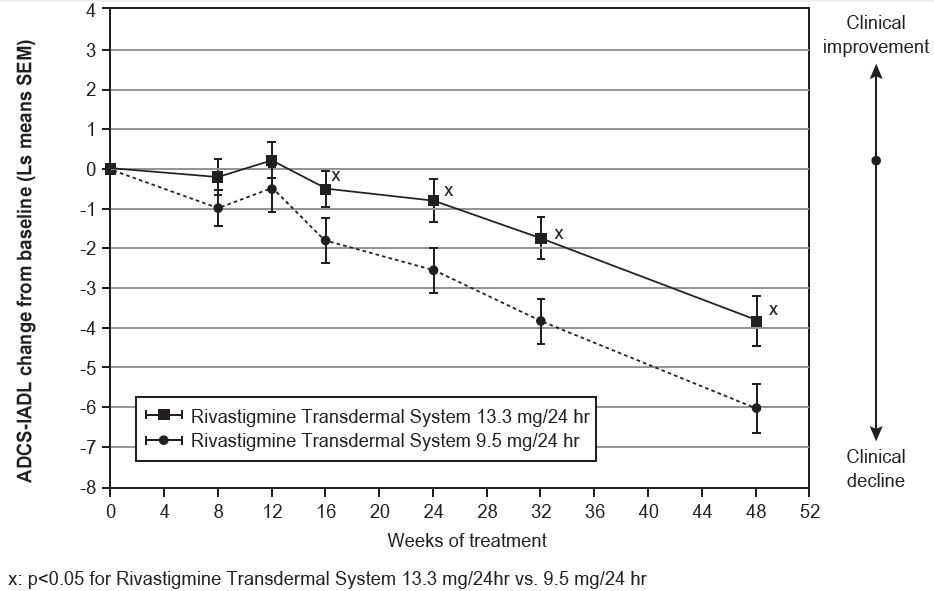
Out of a total of 1584 patients enrolled in the initial open-label phase of the study, 567 patients were classified as decliners and were randomized into the 48-week double-blind treatment phase of the study. Two hundred eighty-seven (287) patients entered the 9.5 mg/24 hours rivastigmine transdermal system treatment group and 280 patients entered the 13.3 mg/24 hours rivastigmine transdermal system treatment group.
Figure 5 illustrates the time course for the mean change from double-blind baseline in ADCS-IADL scores for each treatment group over the course of the 48-week treatment phase of the study. Decline in the mean ADCS-IADL score from the double-blind baseline for the Intent to Treat–Last Observation Carried Forward (ITT-LOCF) analysis was less at each timepoint in the 13.3 mg/24 hour rivastigmine transdermal system treatment group than in the 9.5 mg/24 hours rivastigmine transdermal system treatment group. The 13.3 mg/24 hours dose was statistically significantly superior to the 9.5 mg/24 hours dose at weeks 16, 24, 32, and 48 (primary endpoint).
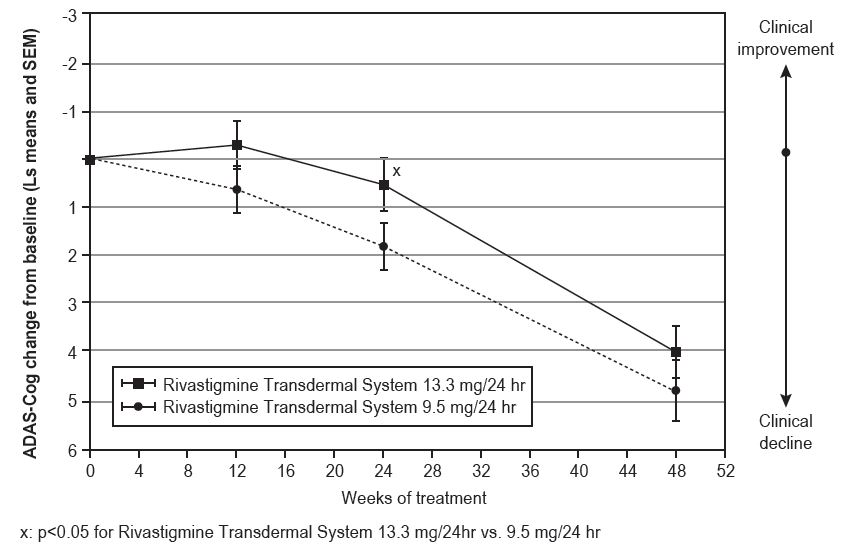
Figure 6 illustrates the time course for the mean change from double-blind baseline in ADAS-Cog scores for both treatment groups over the 48-week treatment phase. The between-treatment group difference for rivastigmine transdermal system 13.3 mg/24 hours versus rivastigmine transdermal system 9.5 mg/24 hours was nominally statistically significant at week 24 (p=0.027), but not at week 48 (p=0.227), which was the primary endpoint.
Figure 5
Time Course of the Change From Double-Blind Baseline in ADCS-IADL Score for Patients Observed at Each Time Point in Study 2
Time Course of the Change From Double-Blind Baseline in ADAS-Cog Score for Patients Observed at Each Time Point in Study 2
24-Week United States Study With Rivastigmine Transdermal System in Severe Alzheimer's Disease (Study 3)
This was a 24-week randomized double-blind, clinical investigation in patients with severe Alzheimer's disease [diagnosed by NINCDS-ADRDA and DSM-IV criteria, Mini-Mental State Examination (MMSE) score greater than or equal to 3 and less than or equal to 12]. The mean age of patients participating in this trial was 78 years with a range of 51 to 96 years with 62% aged greater than 75 years. Approximately 65% of patients were women and 35% were men. The racial distribution was approximately Caucasian 87%, black 7%, Asian 1%, and other races 5%. Patients on a stable dose of memantine were permitted to enter the study. Approximately 61% of the patients in each treatment group were taking memantine throughout the entire duration of the study.
The study was designed to compare the efficacy of rivastigmine transdermal system 13.3 mg/ 24 hours versus that of rivastigmine transdermal system 4.6 mg/24 hours during the 24-week double-blind treatment phase.
The ability of the 13.3 mg/24 hours rivastigmine transdermal system to improve cognitive performance versus that provided by the 4.6 mg/24 hours rivastigmine transdermal system was assessed with the Severe Impairment Battery (SIB) which uses a validated 40-item scale developed for the evaluation of the severity of cognitive dysfunction in more advanced AD patients. The domains assessed included social interaction, memory, language, attention, orientation, praxis, visuospatial ability, construction, and orienting to name. The SIB was scored from 0 to 100, with higher scores reflecting higher levels of cognitive ability.
The ability of the 13.3 mg/ 24 hours rivastigmine transdermal system to improve overall function versus that provided by the 4.6 mg/24 hours rivastigmine transdermal system was assessed with the Alzheimer's Disease Cooperative Study-Activities of Daily Living-Severe Impairment Version (ADCS-ADL-SIV) which is a caregiver-based ADL scale composed of 19 items developed for use in clinical studies of dementia. It is designed to assess the patient's performance of both basic and instrumental activities of daily living such as those necessary for personal care, communicating and interacting with other people, maintaining a household, conducting hobbies and interests, and making judgments and decisions. A sum score is calculated by adding the scores of the individual items and can range from 0 to 54, with higher scores indicating less functional impairment.
In this study, 716 patients were randomized into one of the following treatments: rivastigmine transdermal system 13.3 mg/24 hours or rivastigmine transdermal system 4.6 mg/24 hours in a 1:1 ratio. This 24-week study was divided into an 8-week titration phase followed by a 16-week maintenance phase. In the active treatment arms of this study, temporary dose adjustments below the target dose were permitted during the titration and maintenance phase in the event of poor tolerability.
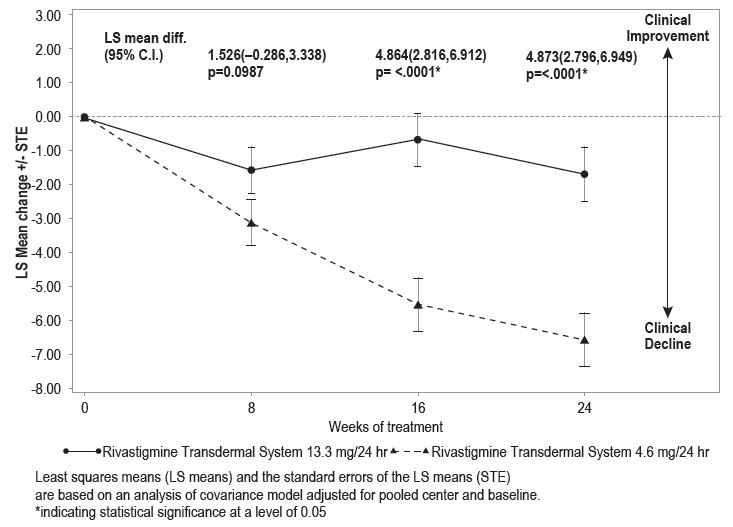
Figure 7 illustrates the time course for the mean change from baseline SIB scores for each treatment group over the course of the 24-week treatment phase of the study. Decline in the mean SIB score from the baseline for the Modified Full Analysis Set (MFAS)-Last Observation Carried Forward (LOCF) analysis was less at each timepoint in the 13.3 mg/24 hours rivastigmine transdermal system treatment group than in the 4.6 mg/24 hours rivastigmine transdermal system treatment group. The 13.3 mg/24 hours dose was statistically significantly superior to the 4.6 mg/24 hours dose at weeks 16 and 24 (primary endpoint).
Figure 8 illustrates the time course for the mean change from baseline in ADCS-ADL-SIV scores for each treatment group over the course of the 24-week treatment phase of the study. Decline in the mean ADCS-ADL-SIV score from baseline for the MFAS-LOCF analysis was less at each timepoint in the 13.3 mg/24 hours rivastigmine transdermal system treatment group than in the 4.6 mg/24 hours rivastigmine transdermal system treatment group. The 13.3 mg/24 hours dose was statistically significantly superior to the 4.6 mg/24 hours dose at weeks 16 and 24 (primary endpoint).
Figure 7
Time Course of the Change From Baseline in SIB Score for Patients Observed at Each Time Point (Modified Full Analysis Set–LOCF)
Time Course of the Change From Baseline in ADCS-ADL-SIV Score for Patients Observed at Each Time Point (Modified Full Analysis Set–LOCF)
Close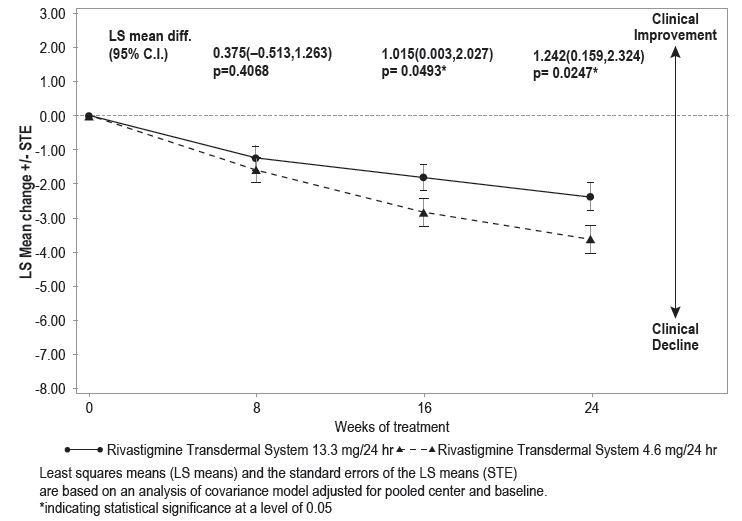
-
16 HOW SUPPLIED/STORAGE AND HANDLINGRivastigmine Transdermal System: 4.6 mg/24 hours - Each transdermal system of 7.75cm2contains 6.975 mg rivastigmine USP with in vivo release rate of 4.6 mg/24 hours. Carton of 30………………………NDC ...
Rivastigmine Transdermal System: 4.6 mg/24 hours
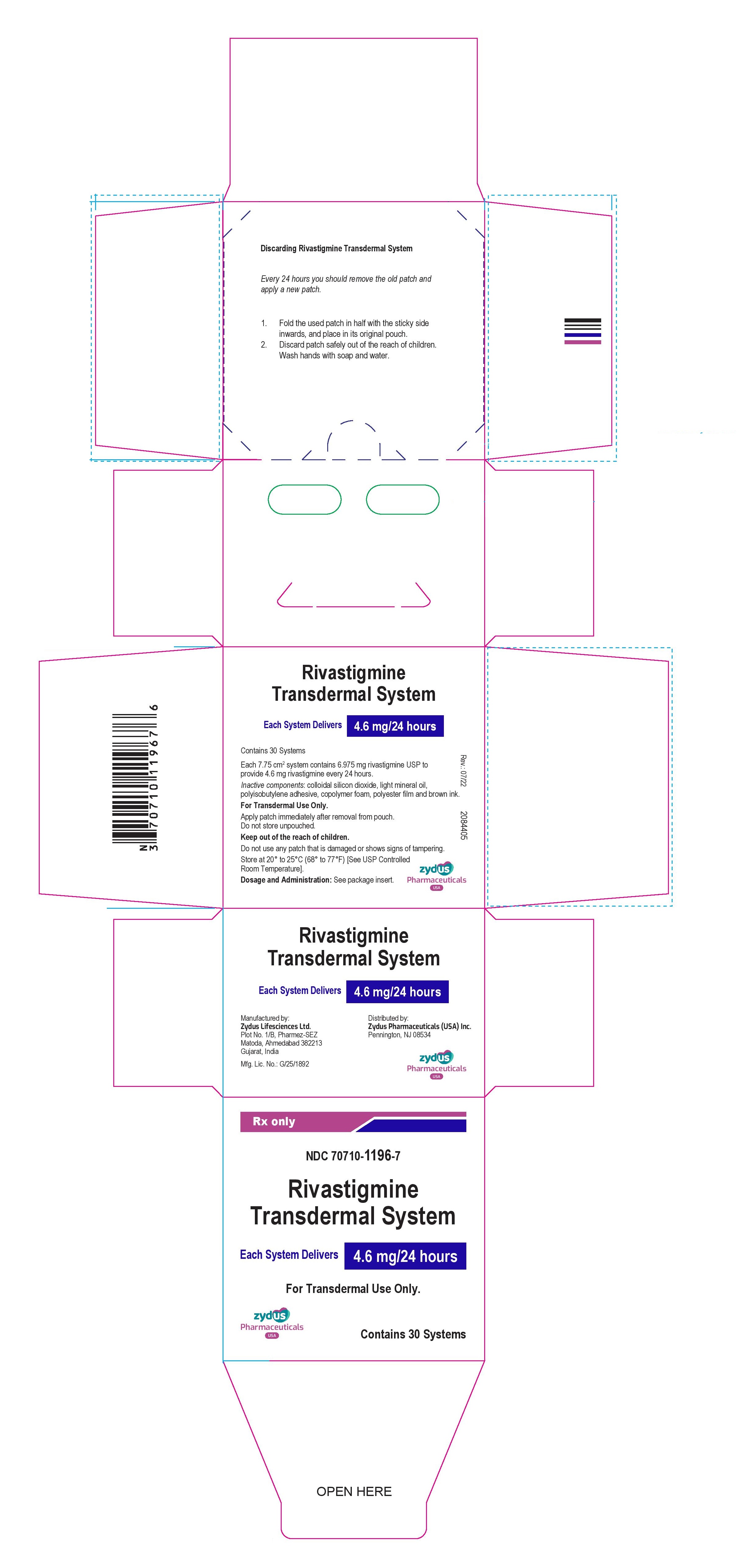
Each transdermal system of 7.75cm2contains 6.975 mg rivastigmine USP with in vivo release rate of 4.6 mg/24 hours.
Carton of 30………………………NDC 70710-1196-7
Rivastigmine Transdermal System: 9.5 mg/24 hours
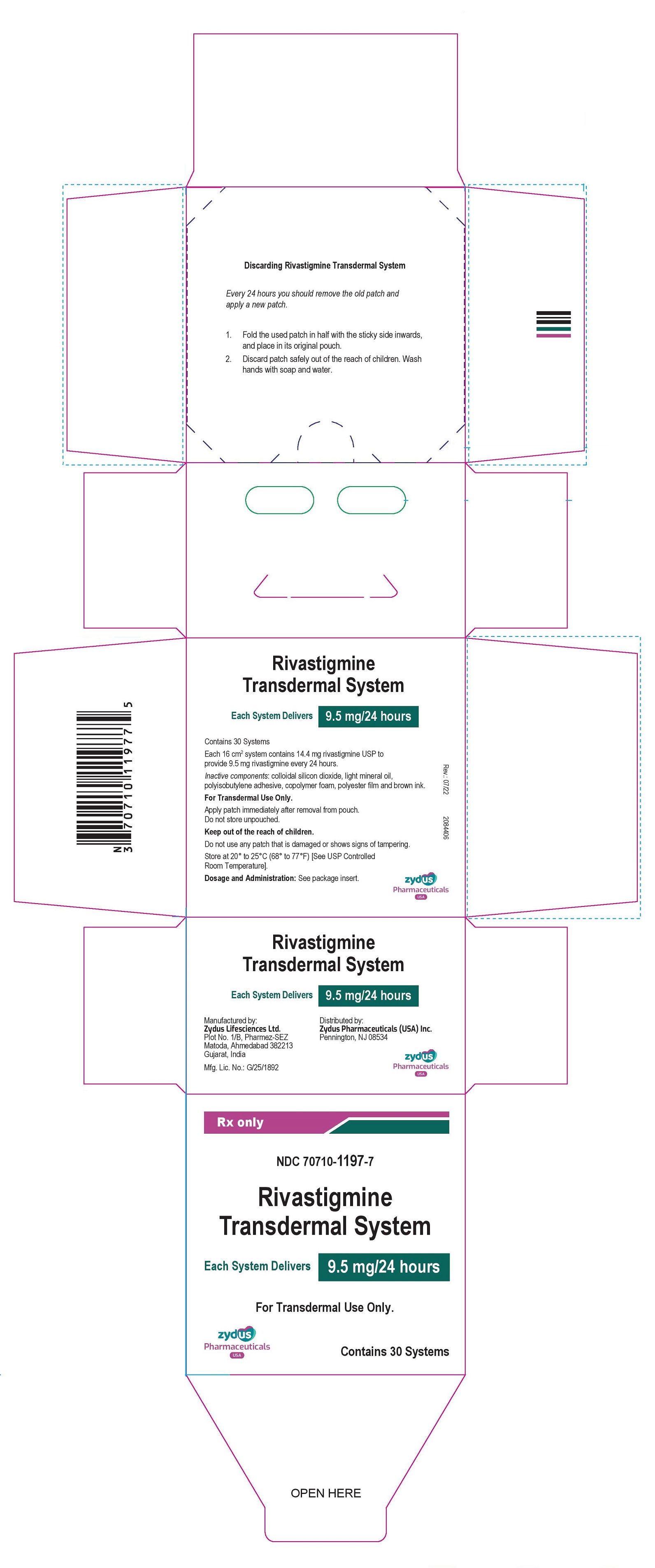
Each transdermal system of 16cm2 contains 14.4 mg rivastigmine USP with in vivo release rate of 9.5 mg/24 hours.
Carton of 30………………………..NDC 70710-1197-7
Rivastigmine Transdermal System: 13.3 mg/24 hours
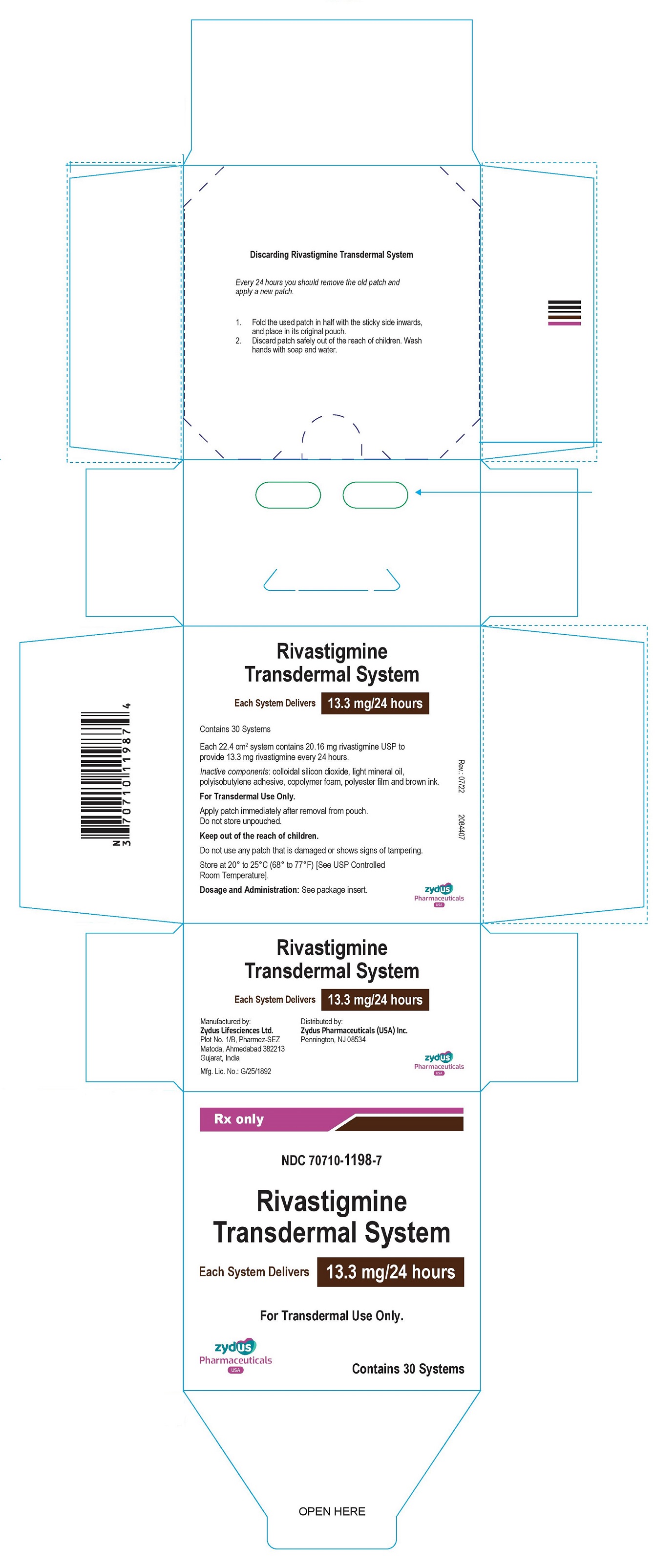
Each transdermal system of 22.4cm2 contains 20.16 mg rivastigmine USP with in vivo release rate of 13.3 mg/24 hours.
Carton of 30………………………..NDC 70710-1198-7
Store at 20° to 25°C (68° to 77°F) [See USP Controlled Room Temperature].
Keep rivastigmine transdermal system in the individual sealed pouch until use. Each pouch contains 1 transdermal system. Used systems should be folded, with the adhesive surfaces pressed together, and discarded safely.
Close -
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use). Importance of Correct Usage - Inform patients or caregivers of the importance of ...
Advise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
Importance of Correct Usage
Inform patients or caregivers of the importance of applying the correct dose on the correct part of the body. They should be instructed to rotate the application site in order to minimize skin irritation. The same site should not be used within 14 days. The previous day's transdermal system must be removed before applying a new transdermal system to a different skin location. Rivastigmine transdermal system should be replaced every 24 hours and the time of day should be consistent. It may be helpful for this to be part of a daily routine, such as the daily bath or shower. Only 1 transdermal system should be worn at a time [see Dosage and Administration (2.4), Warnings and Precautions (5.1)].
Instruct patients or caregivers to avoid exposure of the transdermal system to external heat sources (excessive sunlight, saunas, solariums) for long periods of time.
Instruct patients who have missed a dose to apply a new transdermal system immediately. They may apply the next transdermal system at the usual time the next day. Instruct patients to not apply 2 transdermal systems to make up for 1 missed.
Inform the patient or caregiver to contact the physician for retitration instructions if treatment has been interrupted.
Discarding Used Transdermal Systems
Instruct patients or caregivers to fold the transdermal system in half after use, return the used transdermal system to its original pouch, and discard it out of the reach and sight of children and pets. They should also be informed that drug still remains in the transdermal system after 24-hour usage. They should be instructed to avoid eye contact and to wash their hands after handling the transdermal system. In case of accidental contact with the eyes, or if their eyes become red after handling the transdermal system, they should be instructed to rinse immediately with plenty of water and to seek medical advice if symptoms do not resolve [see Dosage and Administration (2.4)].
Gastrointestinal Adverse Reactions
Inform patients or caregivers of the potential gastrointestinal adverse reactions such as nausea, vomiting, and diarrhea, including the possibility of dehydration due to these symptoms. Explain that rivastigmine transdermal system may affect the patient's appetite and/or the patient's weight. Patients and caregivers should be instructed to look for these adverse reactions, in particular when treatment is initiated or the dose is increased. Instruct patients and caregivers to inform a physician if these adverse reactions persist [see Warnings and Precautions (5.2)].
Skin Reactions
Inform patients or caregivers about the potential for allergic contact dermatitis reactions to occur. Patients or caregivers should be instructed to inform a physician if application-site reactions spread beyond the transdermal system size, if there is evidence of a more intense local reaction (e.g., increasing erythema, edema, papules, vesicles) and if symptoms do not significantly improve within 48 hours after transdermal system removal [see Warnings and Precautions (5.3)].
Concomitant Use of Drugs With Cholinergic Action
Inform patients or caregivers that while wearing rivastigmine transdermal system, patients should not be taking rivastigmine capsules or Rivastigmine oral solution or other drugs with cholinergic effects [see Warnings and Precautions (5.4)].
Pregnancy
Advise patients to notify their healthcare provider if they are pregnant or plan to become pregnant.
Zydus Lifesciences Ltd.
Plot No. 1/B, Pharmez-SEZ, Matoda
Ahmedabad, India
Distributed by:
Zydus Pharmaceuticals (USA) Inc.
Pennington, NJ 08534
Rev.: 05/24
Close -
Patient InformationRivastigmine Transdermal System - (Riv-ah-stig-meen ) What is the most important information I should know about Rivastigmine Transdermal System? Rivastigmine transdermal system is ...Close
Rivastigmine Transdermal System
(Riv-ah-stig-meen )
What is the most important information I should know about Rivastigmine Transdermal System?
Rivastigmine transdermal system is for skin use only.
What is rivastigmine transdermal system?
Rivastigmine transdermal system is a prescription medicine used to treat:
● Mild, moderate, and severe memory problems (dementia) associated with Alzheimer's disease.
● Mild-to-moderate memory problems (dementia) associated with Parkinson's disease (PD).
Based on clinical trials conducted over 6 to 12 months rivastigmine transdermal system was shown to help with cognition which includes (memory, understanding communication, and reasoning) and with doing daily tasks. Rivastigmine transdermal system does not work the same in all people. Some people treated with rivastigmine transdermal system may:
● Seem much better
● Get better in small ways or stay the same
● Get worse but slower than expected
● Not change and then get worse as expected
Some patients will not benefit from treatment with rivastigmine transdermal system. Rivastigmine transdermal system does not cure Alzheimer's disease. All patients with Alzheimer's disease get worse over time.
Rivastigmine transdermal system comes as a transdermal system that delivers rivastigmine (the medicine in rivastigmine transdermal system) through the skin.
It is not known if rivastigmine transdermal system is safe or effective in children under 18 years of age.
Who should not use rivastigmine transdermal system?
Do not use rivastigmine transdermal system if you:
● are allergic to rivastigmine, carbamate derivatives, or any of the ingredients in rivastigmine transdermal system. See the end of this leaflet for a complete list of ingredients in rivastigmine transdermal system.
● have had a skin reaction that:
○ spread beyond the rivastigmine transdermal system size
○ had blisters, increased skin redness, or swelling
○ did not get better within 48 hours after you removed the rivastigmine transdermal system
Ask your healthcare provider if you are not sure if you should use rivastigmine transdermal system.
What should I tell my healthcare provider before using rivastigmine transdermal system?
Before you use rivastigmine transdermal system, tell your healthcare provider if you:
● have or have had a stomach ulcer
● are planning to have surgery
● have or have had problems with your heart
● have problems passing urine
● have or have had seizures
● have problems with movement (tremors)
● have asthma or breathing problems
● have a loss of appetite or are losing weight
● have had a skin reaction to rivastigmine (the medicine in rivastigmine transdermal system) in the past
● have any other medical conditions
● are pregnant or plan to become pregnant. It is not known if the medicine in rivastigmine transdermal system will harm your unborn baby. Talk to your healthcare provider if you are pregnant or plan to become pregnant.
● are breastfeeding or plan to breastfeed. It is not known if the medicine in rivastigmine transdermal system passes into your breast milk. Talk to your doctor about the best way to feed your baby if you take rivastigmine transdermal system.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
Especially tell your healthcare provider if you take:
● a medicine used to treat inflammation [nonsteroidal anti-inflammatory drugs (NSAIDs)]
● other medicines used to treat Alzheimer's or Parkinson's disease
● an anticholinergic medicine, such as an allergy or cold medicine, a medicine to treat bladder or bowel spasms, or certain asthma medicines, or certain medicines to prevent motion or travel sickness
● metoclopramide, a drug given to relieve symptoms of nausea, gastroesophageal reflux disease(GERD), or nausea and vomiting after surgery or chemotherapy treatment
● If you are undergoing surgery while using rivastigmine transdermal system, inform your doctor because rivastigmine transdermal system may exaggerate the effects of anesthesia, or the effects of a beta-blocker, a type of medicine given for high blood pressure, heart disease, and other medical conditions
Ask your healthcare provider if you are not sure if your medicine is one listed above. Know the medicines you take. Keep a list of them to show to your healthcare provider and pharmacist when you get a new medicine.
How should I use rivastigmine transdermal system?
● Use rivastigmine transdermal system exactly as your healthcare provider tells you to use it.
● Rivastigmine transdermal system comes in 3 different dosage strengths.
● Your healthcare provider may change your dose as needed.
● Wear only 1 rivastigmine transdermal system at a time.
● Rivastigmine transdermal system is for skin use only.
● Only apply rivastigmine transdermal system to healthy skin that is clean, dry, hairless, and free of redness, irritation, burns or cuts.
● Avoid applying rivastigmine transdermal system to areas on your body that will be rubbed against tight clothing.
● Do not apply rivastigmine transdermal system to skin that has cream, lotion, or powder on it.
● Change your rivastigmine transdermal system every 24 hours at the same time of day. You may write the date and time you put on the rivastigmine transdermal system with a ballpoint pen before applying the transdermal system to help you remember when to remove it.
● Change your application site every day to avoid skin irritation. You can use the same area, but do not use the exact same spot for at least 14 days after your last application.
● Check to see if the rivastigmine transdermal system has become loose when you are bathing, swimming, or showering.
● Rivastigmine transdermal system is designed to deliver medication during the time it is worn. If your rivastigmine transdermal system falls off before its usual replacement time, put on a new rivastigmine transdermal system right away. Replace the new transdermal system the next day at the same time as usual. Do not use overlays, bandages, or tape to secure a rivastigmine transdermal system that has become loose or try to reapply a rivastigmine transdermal system that has fallen off.
● If you miss a dose or forget to change your rivastigmine transdermal system apply your next rivastigmine transdermal system as soon as you remember. Do not apply 2 rivastigmine transdermal systems to make up for the missed dose.
● If you miss more than 3 doses of applying rivastigmine transdermal system, call your healthcare provider before putting on a new rivastigmine transdermal system. You may need to restart rivastigmine transdermal system at a lower dose.
● Always remove the old rivastigmine transdermal system from the previous day before you apply a new one.
● Having more than 1 rivastigmine transdermal system on your body at the same time can cause you to get too much medicine. If you accidentally use more than 1 rivastigmine transdermal system at a time, call your healthcare provider right away. If you are unable to reach your healthcare provider, call your local Poison Control Center at 1-800-222-1222 or go to the nearest hospital emergency room right away.
What should I avoid while using rivastigmine transdermal system?
● Do not touch your eyes after you touch the rivastigmine transdermal system. In case of accidental contact with your eyes or if your eyes become red after handling the transdermal system, rinse immediately with plenty of water and seek medical advice if symptoms do not resolve.
● Rivastigmine transdermal system can cause drowsiness, dizziness, weakness, or fainting. Do not drive, operate heavy machinery, or do other dangerous activities until you know how rivastigmine transdermal system affects you.
● Avoid exposure to heat sources such as excessive sunlight, saunas, or sunrooms for long periods of time.
What are the possible side effects of rivastigmine transdermal system?
Rivastigmine transdermal system may cause serious side effects, including:
● Medication overdose . Hospitalization and rarely death may happen when people accidently wear more than 1 transdermal system at the same time. It is important that the old rivastigmine transdermal system be removed before you apply a new one. Do not wear more than 1 rivastigmine transdermal system at a time.
● Stomach or bowel (intestinal) problems, including :
○ nausea
○ vomiting
○ diarrhea
○ dehydration
○ loss of appetite
○ weight loss
○ bleeding in your stomach (ulcers)
● Skin reactions . Some people have had a serious skin reaction called allergic contact dermatitis (ACD) when using rivastigmine transdermal system. Stop using rivastigmine transdermal system and call your healthcare provider right away if you experience reactions that spread beyond the transdermal system size, are intense in nature and do not improve within 48 hours after the transdermal system is removed. Symptoms of ACD may be intense and include:
○ itching, redness, swelling, warmth or tenderness of the skin
○ peeling or blistering of the skin that may ooze, drain or crust over
● Heart problems
● Seizures
● Problems with movement (tremors)
The most common side effects of rivastigmine transdermal systeminclude:
○ depression
○ headache
○ anxiety
○ dizziness
○ stomach pain
○ urinary tract infections
○ muscle weakness
○ tiredness
○ trouble sleeping
Tell your healthcare provider if you have any side effect that bothers you or that does not go away.
These are not all the possible side effects of rivastigmine transdermal system. For more information, ask your healthcare provider or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to the FDA at 1-800-FDA-1088.
How should I store rivastigmine transdermal system?
○ Store rivastigmine transdermal system between 68ºF to 77ºF (20ºC to 25ºC).
○ Keep rivastigmine transdermal system in the sealed pouch until ready to use.
Keep rivastigmine transdermal system and all medicines out of the reach of children .
General information about the safe and effective use of rivastigmine transdermal system .
Medicines are sometimes prescribed for purposes other than those listed in the Patient Information leaflet. Do not use rivastigmine transdermal system for a condition for which it was not prescribed. Do not give rivastigmine transdermal system to other people, even if they have the same symptoms you have. It may harm them.
This Patient Information leaflet summarizes the most important information about rivastigmine transdermal system. If you would like more information, talk with your healthcare provider. You can ask your pharmacist or healthcare provider for information about rivastigmine transdermal system that is written for health professionals.
For more information, go to www.zydususa.com or call 1-877-993-8779.
What are the ingredients of rivastigmine transdermal system?
Active ingredient: rivastigmine USP
Excipients include : colloidal silicon dioxide, light mineral oil, polyisobutylene adhesive, copolymer foam and polyester film. Additionally, rivastigmine transdermal system is printed with pharmaceutical grade brown ink which contains acrylic polymers, carbon black, ethoxylated 2,4,7,9-tetramethyl 5 decyn-4,7-diol, iron oxide, octylphenoxypolyethoxyethanol, polyethylene glycol, polyethylene wax and polytetrafluoroethylene.
-
Instructions for UseRivastigmine Transdermal System - (Riv-ah-stig-meen) You will need the following supplies (See Figure A): Rivastigmine transdermal system is supplied in cartons containing 30 transdermal ...
Rivastigmine Transdermal System
(Riv-ah-stig-meen)
You will need the following supplies (See Figure A):
Rivastigmine transdermal system is supplied in cartons containing 30 transdermal systems (See Figure A)
Figure A
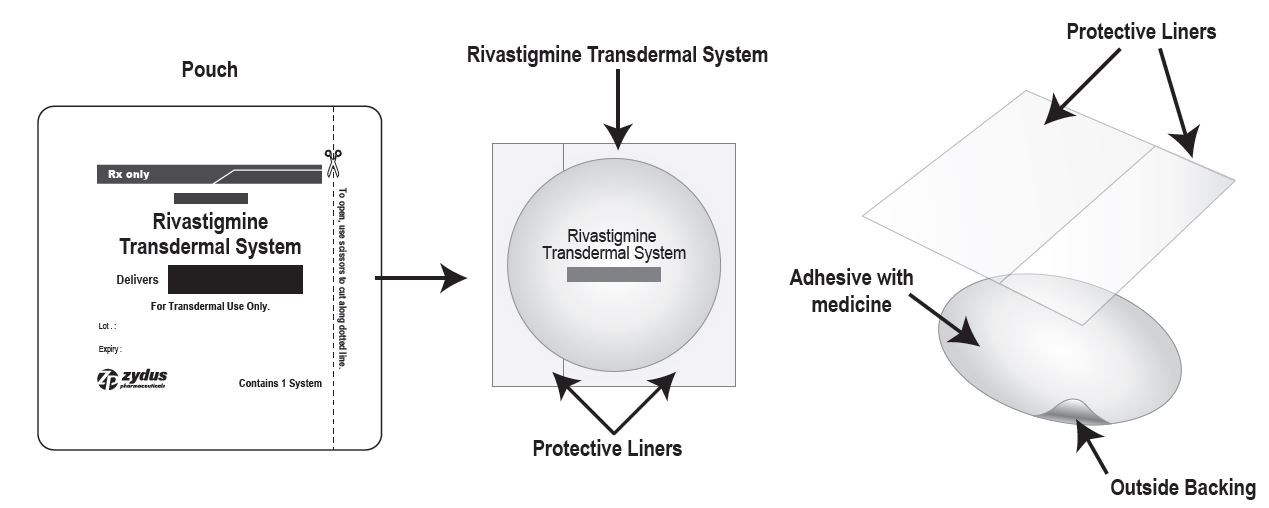
- Rivastigmine transdermal system is a thin, beige, plastic patch that sticks to the skin. Each rivastigmine transdermal system is sealed in a pouch that protects it until you are ready to put it on (See Figure A).
- Only 1 rivastigmine transdermal system should be worn at a time. Do not apply more than 1 rivastigmine transdermal system at a time to the body.
- Do not open the pouch or remove the rivastigmine transdermal system until you are ready to apply it.
Using rivastigmine transdermal system:
Step 1. Choose an area to apply the rivastigmine transdermal system (See Figure B).
- Instructions for Caregivers: Apply rivastigmine transdermal system to the upper or lower back if it is likely that the patient will remove it. If this is not a concern, the rivastigmine transdermal system can be applied insteadto the upper arm orchest. Do not apply the rivastigmine transdermal system to areas where it can be rubbed off by tight clothing or belts.
- Only apply the rivastigmine transdermal system to healthy skin that is clean, dry, hairless, and free of redness, irritation, burns or cuts.
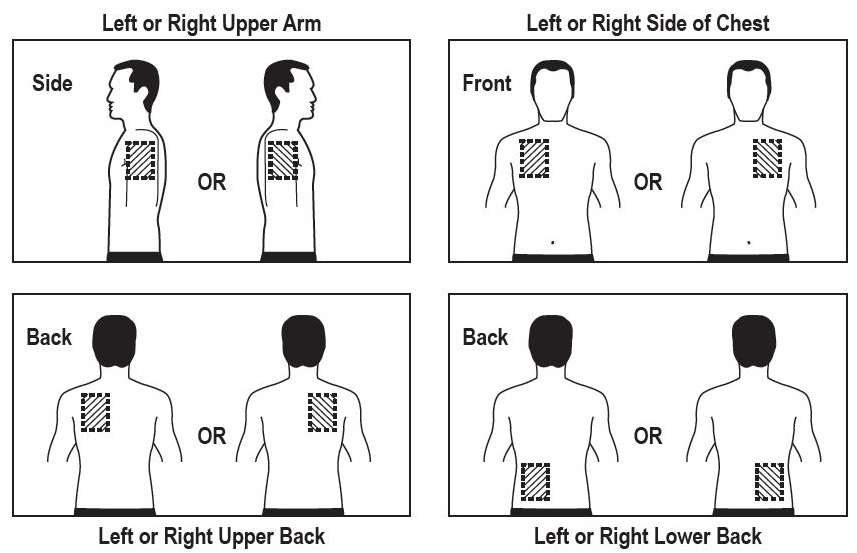
The diagram represents areas on the body where rivastigmine transdermal system may be applied. Only 1 transdermal system should be worn at a time. Do not apply multiple
transdermal systems to the body.
Step 2. Remove the rivastigmine transdermal system from the pouch (See Figure C).
Carefully cut the pouch along the dotted line to open and remove the rivastigmine transdermal system. Save the pouch for later use.
Figure C
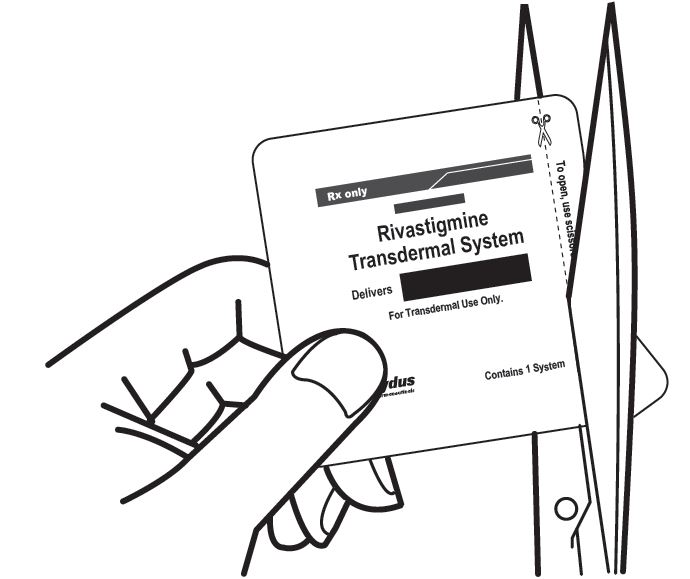
- Do not cut or fold the rivastigmine transdermal system itself.
Step 3. Hold your rivastigmine transdermal system (See Figure D).
- Hold your rivastigmine transdermal system with both hands, with the protective liner on top and bend the edges of your transdermal system away from you.
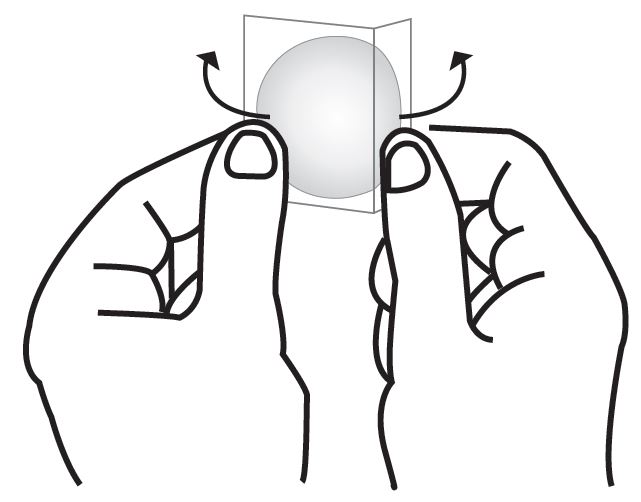
Step 4. Remove 1 side of the adhesive liner (See Figure E).
- A protective liner covers the sticky (adhesive) side of the rivastigmine transdermal system. Peel off smaller side of the protective cover. Do not touch the sticky part of the rivastigmine transdermal system with your fingers.
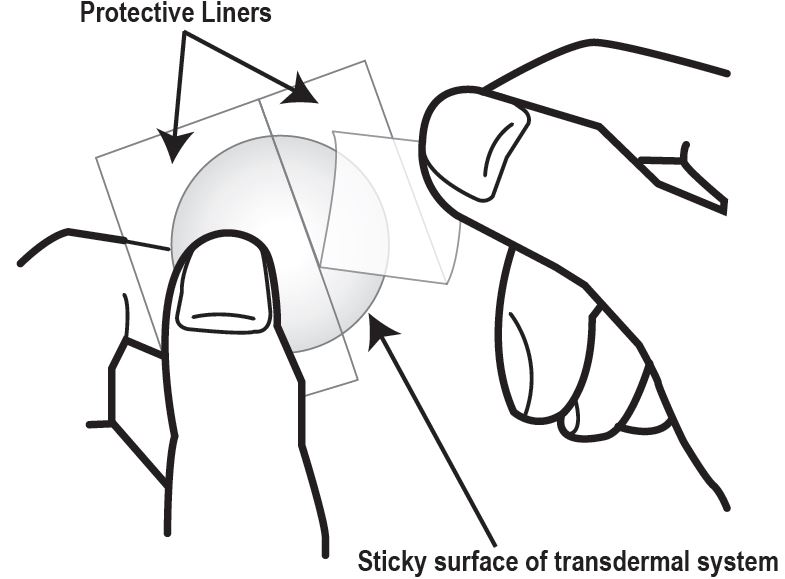
Step 5. Apply the rivastigmine transdermal system to your skin (See Figure F).
- Apply the sticky (adhesive) side of the rivastigmine transdermal system to your chosen area of skin and then peel off the other side of the protective cover.
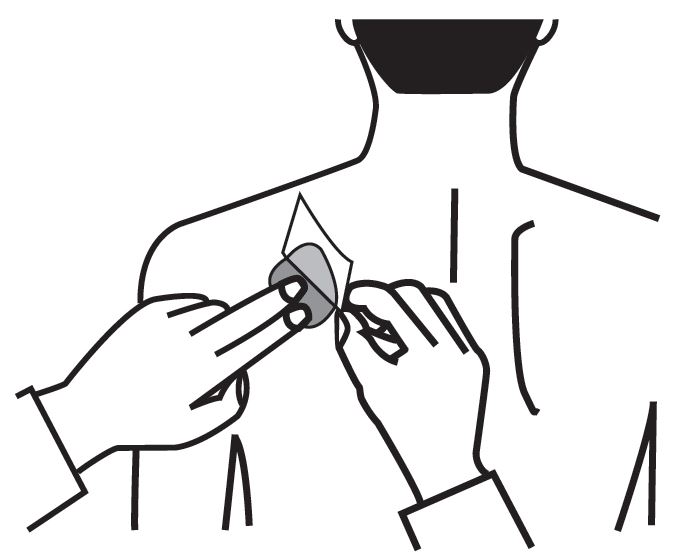
- Press down on the rivastigmine transdermal system firmly for 30 seconds to make sure that the edges stick to your skin (See Figure G).
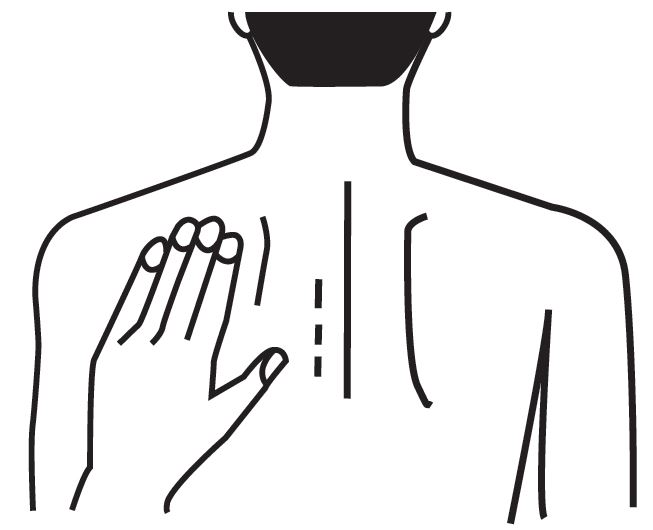
Step 6. Wash your hands with soap and water right away.
Note:
- If your rivastigmine transdermal system falls off, select a new area, and repeat Steps 2 to 6 to apply a new rivastigmine transdermal system.
- Be sure to replace the new rivastigmine transdermal system the next day at the same time as usual.
Removing your rivastigmine transdermal system:
Step 7. Remove the rivastigmine transdermal system from the skin (See Figure H).
- Gently pull on 1 edge of the rivastigmine transdermal system to remove it from your skin.
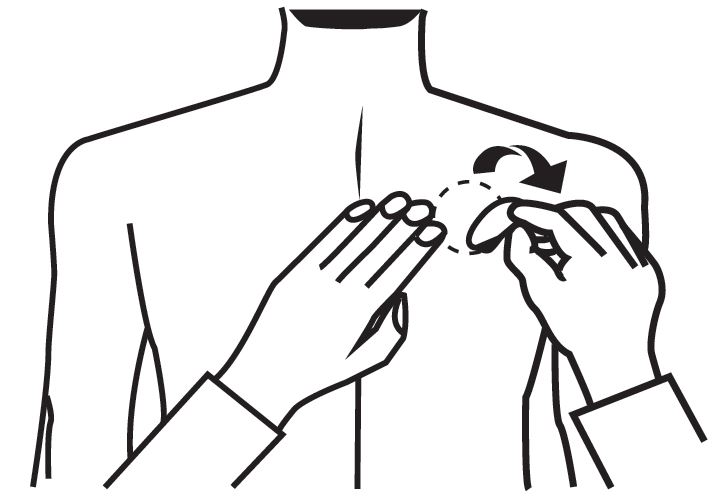
Throwing away the used rivastigmine transdermal system:
Step 8. Throw away the used rivastigmine transdermal system (See Figure I).
- Fold the used rivastigmine transdermal system in half (with the sticky sides together) and put it back into the pouch that you saved.
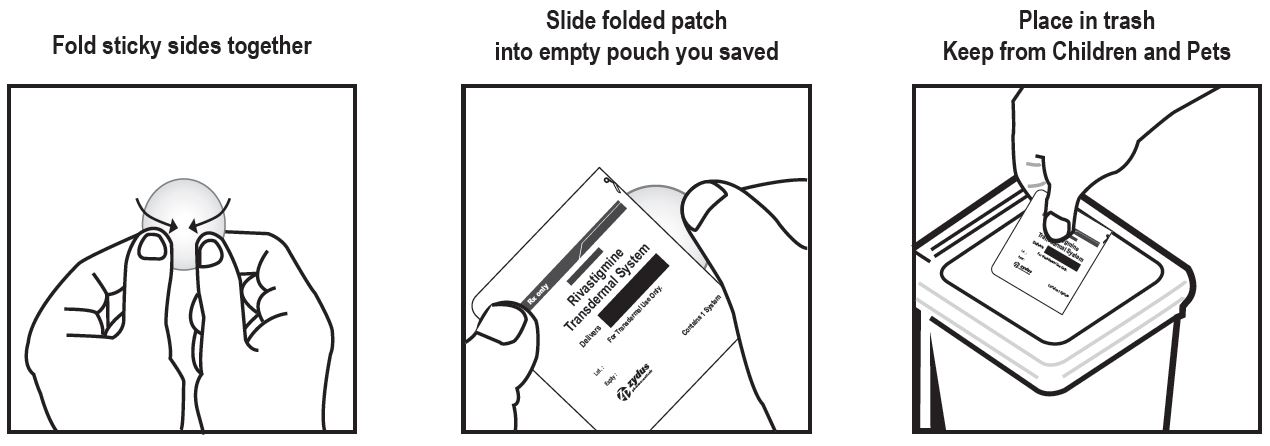
- Throw away the used rivastigmine transdermal system safely and out of the reach of children and pets.
- Some medicine stays in the transdermal system for 24 hours after you use it and should be folded together (sticky side together) and safely thrown away. Do not try to re-use rivastigmine transdermal systems .
Step 9. Wash your hands with soap and water right away.
- After you remove the rivastigmine transdermal system, if any adhesive remains on your skin, you can use soap and water or an oil-based substance (such as baby oil) to remove the adhesive. Alcohol or other dissolving liquids (such as nail polish remover) should not be used.
This Patient Information and Instructions for Use have been approved by the U.S. Food and Drug Administration.
Manufactured by:
Zydus Lifesciences Ltd.
Plot No. 1/B, Pharmez-SEZ, Matoda
Ahmedabad, India
Distributed by:
Zydus Pharmaceuticals (USA) Inc.
Pennington, NJ 08534
Rev.: 07/22
2084408
Close -
PACKAGE LABEL PRINCIPAL DISPLAY PANELRx only - NDC 70710-1196-7 - Rivastigmine - Transdermal System - Each System Delivers 4.6 mg/24 hours - For Transdermal Use Only. Contains 30 systems - zydus - Pharmaceuticals - USA - Rx only - NDC ...
NDC 70710-1196-7
Rivastigmine
Transdermal System
Each System Delivers 4.6 mg/24 hours
For Transdermal Use Only.
Contains 30 systems
zydus
Pharmaceuticals
USA
NDC 70710-1197-7
Rivastigmine
Transdermal System
Each System Delivers 9.5 mg/24 hours
For Transdermal Use Only.
Contains 30 systems
zydus
Pharmaceuticals
USA
NDC 70710-1198-7
Rivastigmine
Transdermal System
Each System Delivers 13.3 mg/24 hours
For Transdermal Use Only.
Contains 30 systems
zydus
Pharmaceuticals
USA
Close
-
INGREDIENTS AND APPEARANCEProduct Information
RIVASTIGMINE rivastigmine patch, extended release Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:70710-1196 Route of Administration TRANSDERMAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength RIVASTIGMINE (UNII: PKI06M3IW0) (RIVASTIGMINE - UNII:PKI06M3IW0) RIVASTIGMINE 4.6 mg in 24 h Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) LIGHT MINERAL OIL (UNII: N6K5787QVP) POLYISOBUTYLENE (1100000 MW) (UNII: FLT10CH37X) POLYISOBUTYLENE (55000 MW) (UNII: TQ77WR8A02) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70710-1196-7 30 in 1 CARTON 03/06/2019 1 NDC:70710-1196-1 24 h in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA206318 03/06/2019 RIVASTIGMINE rivastigmine patch, extended release Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:70710-1197 Route of Administration TRANSDERMAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength RIVASTIGMINE (UNII: PKI06M3IW0) (RIVASTIGMINE - UNII:PKI06M3IW0) RIVASTIGMINE 9.5 mg in 24 h Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) LIGHT MINERAL OIL (UNII: N6K5787QVP) POLYISOBUTYLENE (1100000 MW) (UNII: FLT10CH37X) POLYISOBUTYLENE (55000 MW) (UNII: TQ77WR8A02) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70710-1197-7 30 in 1 CARTON 03/06/2019 1 NDC:70710-1197-1 24 h in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA206318 03/06/2019 RIVASTIGMINE rivastigmine patch, extended release Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:70710-1198 Route of Administration TRANSDERMAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength RIVASTIGMINE (UNII: PKI06M3IW0) (RIVASTIGMINE - UNII:PKI06M3IW0) RIVASTIGMINE 13.3 mg in 24 h Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) LIGHT MINERAL OIL (UNII: N6K5787QVP) POLYISOBUTYLENE (1100000 MW) (UNII: FLT10CH37X) POLYISOBUTYLENE (55000 MW) (UNII: TQ77WR8A02) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70710-1198-7 30 in 1 CARTON 03/06/2019 1 NDC:70710-1198-1 24 h in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA206318 03/06/2019 Labeler - Zydus Pharmaceuticals USA Inc. (156861945) Registrant - Zydus Pharmaceuticals USA Inc. (156861945)
CloseEstablishment Name Address ID/FEI Business Operations Zydus Lifesciences Limited 650461283 ANALYSIS(70710-1196, 70710-1197, 70710-1198) , MANUFACTURE(70710-1196, 70710-1197, 70710-1198)
Find additional resources
(also available in the left menu)Safety
Report Adverse Events, FDA Safety Recalls, Presence in Breast Milk
Related Resources
Medline Plus, Clinical Trials, PubMed, Biochemical Data Summary
More Info on this Drug
View Labeling Archives, RxNorm, Get Label RSS Feed, View NDC Code(s)NEW!
View Labeling Archives for this drug
RIVASTIGMINE patch, extended release
Number of versions: 4
| Published Date (What is this?) | Version | Files |
|---|---|---|
| Jun 3, 2024 | 4 (current) | download |
| Oct 5, 2022 | 3 | download |
| Dec 7, 2020 | 2 | download |
| Mar 6, 2019 | 1 | download |
RxNorm
RIVASTIGMINE patch, extended release
| RxCUI | RxNorm NAME | RxTTY | |
|---|---|---|---|
| 1 | 725021 | rivastigmine 4.6 MG/Day 24HR Transdermal System | PSN |
| 2 | 725021 | 24 HR rivastigmine 0.192 MG/HR Transdermal System | SCD |
| 3 | 725021 | rivastigmine 0.192 MG/HR 24 HR Transdermal Patch | SY |
| 4 | 725021 | rivastigmine 4.6 MG/Day 24 HR Transdermal Patch | SY |
| 5 | 725021 | rivastigmine 4.6 MG/Day 24HR Transdermal System | SY |
| 6 | 725023 | rivastigmine 9.5 MG/Day 24HR Transdermal System | PSN |
| 7 | 725023 | 24 HR rivastigmine 0.396 MG/HR Transdermal System | SCD |
| 8 | 725023 | rivastigmine 0.396 MG/HR 24 HR Transdermal Patch | SY |
| 9 | 725023 | rivastigmine 9.5 MG/Day 24 HR Transdermal Patch | SY |
| 10 | 725023 | rivastigmine 9.5 MG/Day 24HR Transdermal System | SY |
| 11 | 1308569 | rivastigmine 13.3 MG/Day 24HR Transdermal System | PSN |
| 12 | 1308569 | 24 HR rivastigmine 0.554 MG/HR Transdermal System | SCD |
| 13 | 1308569 | rivastigmine 13.3 MG/Day 24HR Transdermal Patch | SY |
| 14 | 1308569 | rivastigmine 13.3 MG/Day 24HR Transdermal System | SY |
Get Label RSS Feed for this Drug
RIVASTIGMINE patch, extended release
To receive this label RSS feed
Copy the URL below and paste it into your RSS Reader application.
https://dailymed.nlm.nih.gov/dailymed/labelrss.cfm?setid=64dad603-2389-456b-8c18-d1d40b7e7419
To receive all DailyMed Updates for the last seven days
Copy the URL below and paste it into your RSS Reader application.
https://dailymed.nlm.nih.gov/dailymed/rss.cfm
What will I get with the DailyMed RSS feed?
DailyMed will deliver notification of updates and additions to Drug Label information currently shown on this site through its RSS feed.
DailyMed will deliver this notification to your desktop, Web browser, or e-mail depending on the RSS Reader you select to use. To view updated drug label links, paste the RSS feed address (URL) shown below into a RSS reader, or use a browser which supports RSS feeds, such as Safari for Mac OS X.
How to discontinue the RSS feed
If you no longer wish to have this DailyMed RSS service, simply delete the copied URL from your RSS Reader.
More about getting RSS News & Updates from DailyMedWhy is DailyMed no longer displaying pill images on the Search Results and Drug Info pages?
Due to inconsistencies between the drug labels on DailyMed and the pill images provided by RxImage, we no longer display the RxImage pill images associated with drug labels.
We anticipate reposting the images once we are able identify and filter out images that do not match the information provided in the drug labels.
NDC Codes
RIVASTIGMINE patch, extended release
If this SPL contains inactivated NDCs listed by the FDA initiated compliance action, they will be specified as such.
| NDC | |
|---|---|
| 1 | 70710-1196-1 |
| 2 | 70710-1196-7 |
| 3 | 70710-1197-1 |
| 4 | 70710-1197-7 |
| 5 | 70710-1198-1 |
| 6 | 70710-1198-7 |