Label: BETAMETHASONE VALERATE- betamethasone valerate foam aerosol, foam
- NDC Code(s): 70700-141-19, 70700-141-20
- Packager: Xiromed, LLC.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated May 8, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONRx Only - For Dermatologic Use Only - Not for Ophthalmic Use
-
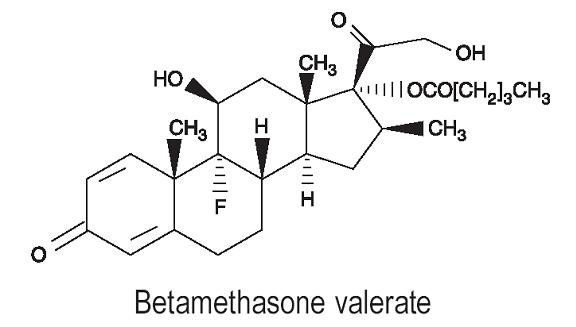
DESCRIPTIONBetamethasone valerate foam, 0.12% contains betamethasone valerate, USP, a synthetic corticosteroid, for topical dermatologic use. The corticosteroids constitute a class of primarily synthetic ...
-
CLINICAL PHARMACOLOGYLike other topical corticosteroids, betamethasone valerate foam has anti-inflammatory, antipruritic, and vasoconstrictive properties. The mechanism of the anti-inflammatory activity of the topical ...
-
CLINICAL STUDIESThe safety and efficacy of betamethasone valerate foam, 0.12% has been demonstrated in a four-week trial. An adequate and well-controlled clinical trial was conducted in 190 patients with moderate ...
-
INDICATIONS AND USAGEBetamethasone valerate foam, 0.12% is a medium potency topical corticosteroid indicated for relief of the inflammatory and pruritic manifestations of corticosteroid-responsive dermatoses of the ...
-
CONTRAINDICATIONSBetamethasone valerate foam, 0.12% is contraindicated in patients who are hypersensitive to betamethasone valerate, to other corticosteroids, or to any ingredient in this preparation.
-
PRECAUTIONSGeneral - Systemic absorption of topical corticosteroids has caused reversible hypothalamic-pituitary-adrenal (HPA) axis suppression with the potential for glucocorticosteroid insufficiency after ...
-
ADVERSE REACTIONSThe most frequent adverse event was burning/itching/stinging at the application site; the incidence and severity of this event were as follows: Incidence and severity of ...
-
OVERDOSAGETopically applied betamethasone valerate foam, 0.12% can be absorbed in sufficient amounts to produce systemic effects. (See PRECAUTIONS)
-
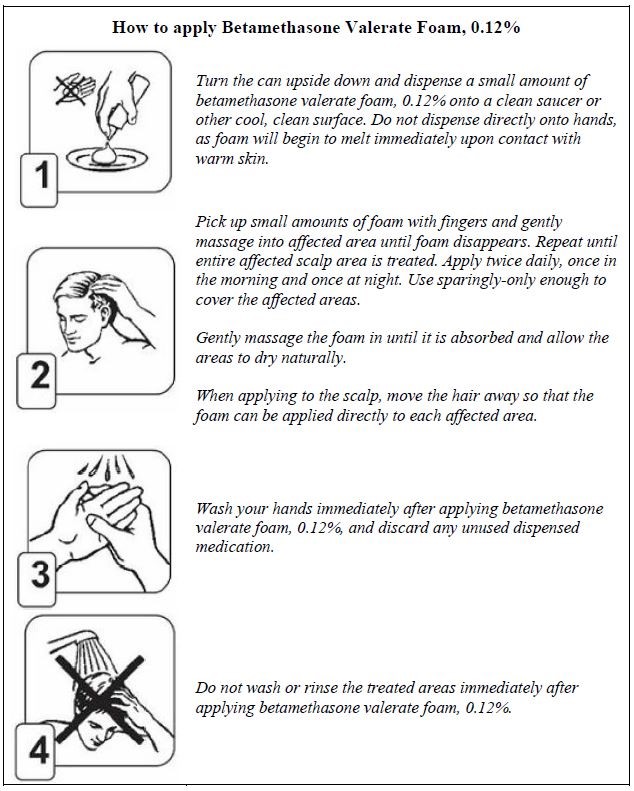
DOSAGE AND ADMINISTRATIONNote: For proper dispensing of foam, can must be inverted. For application to the scalp invert can and dispense a small amount of betamethasone valerate foam, 0.12% onto a saucer or other cool ...
-
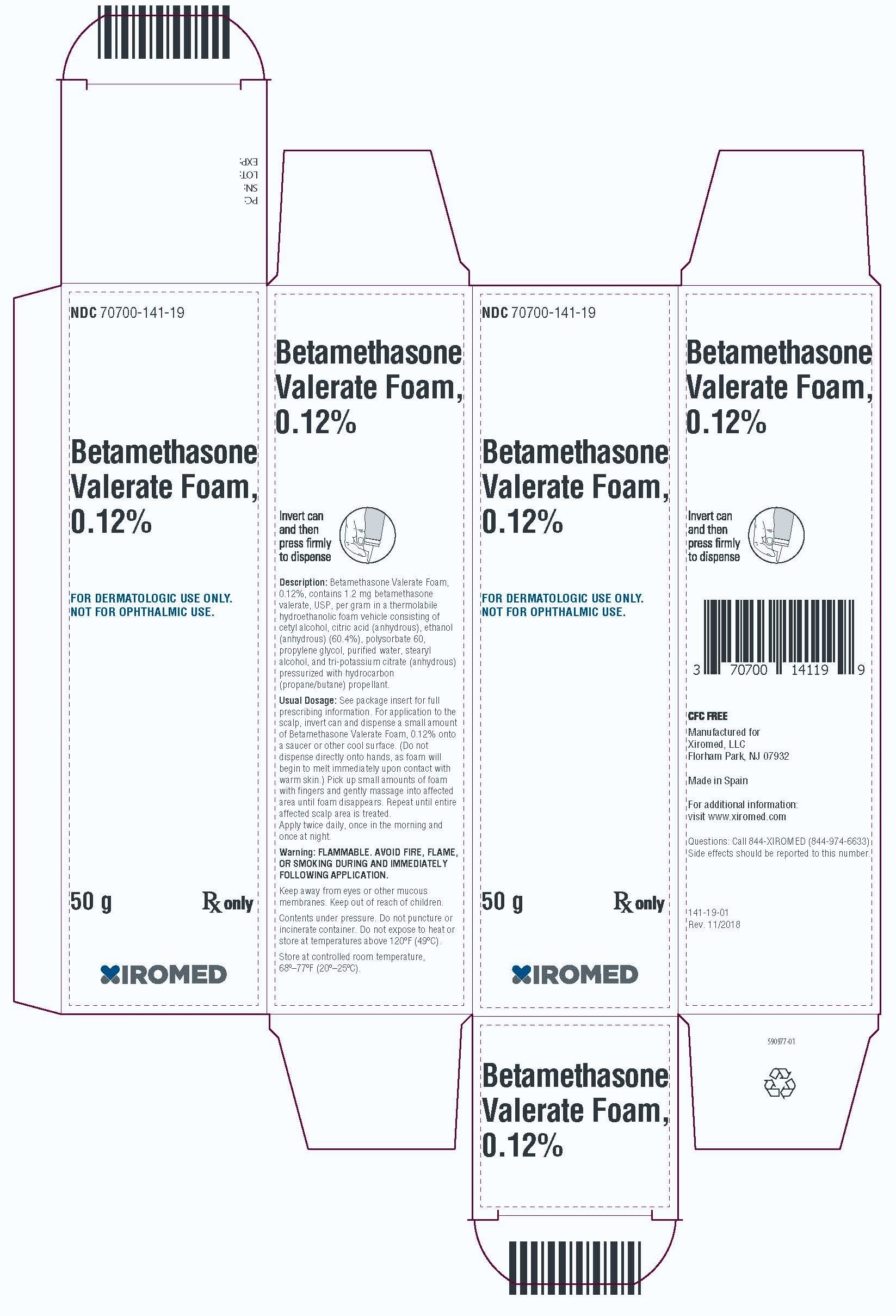
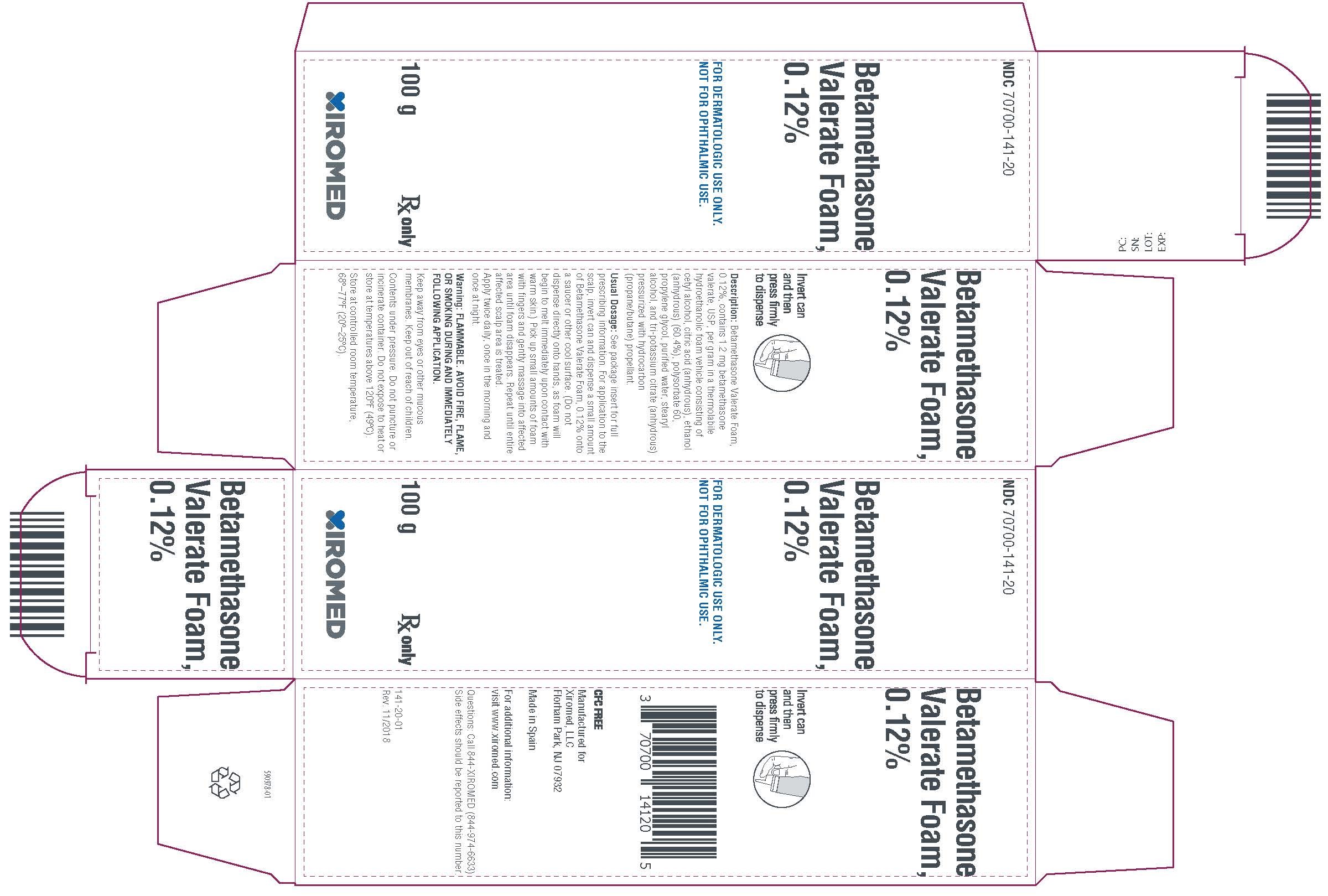
HOW SUPPLIEDBetamethasone valerate foam, 0.12% is supplied as follows: 50 g aluminum can NDC 70700-141-19 - 100 g aluminum can NDC 70700-141-20 - Store at ...
-
WARNINGFLAMMABLE. AVOID FIRE, FLAME OR SMOKING DURING AND IMMEDIATELY FOLLOWING APPLICATION. Keep out of reach of children. Contents under pressure. Do not puncture or incinerate container. Do not ...
-
PATIENT INFORMATIONBetamethasone valerate foam, 0.12% About betamethasone valerate foam, 0.12% Your doctor has prescribed betamethasone valerate foam, 0.12%, for the relief of corticosteroid-responsive skin ...
-
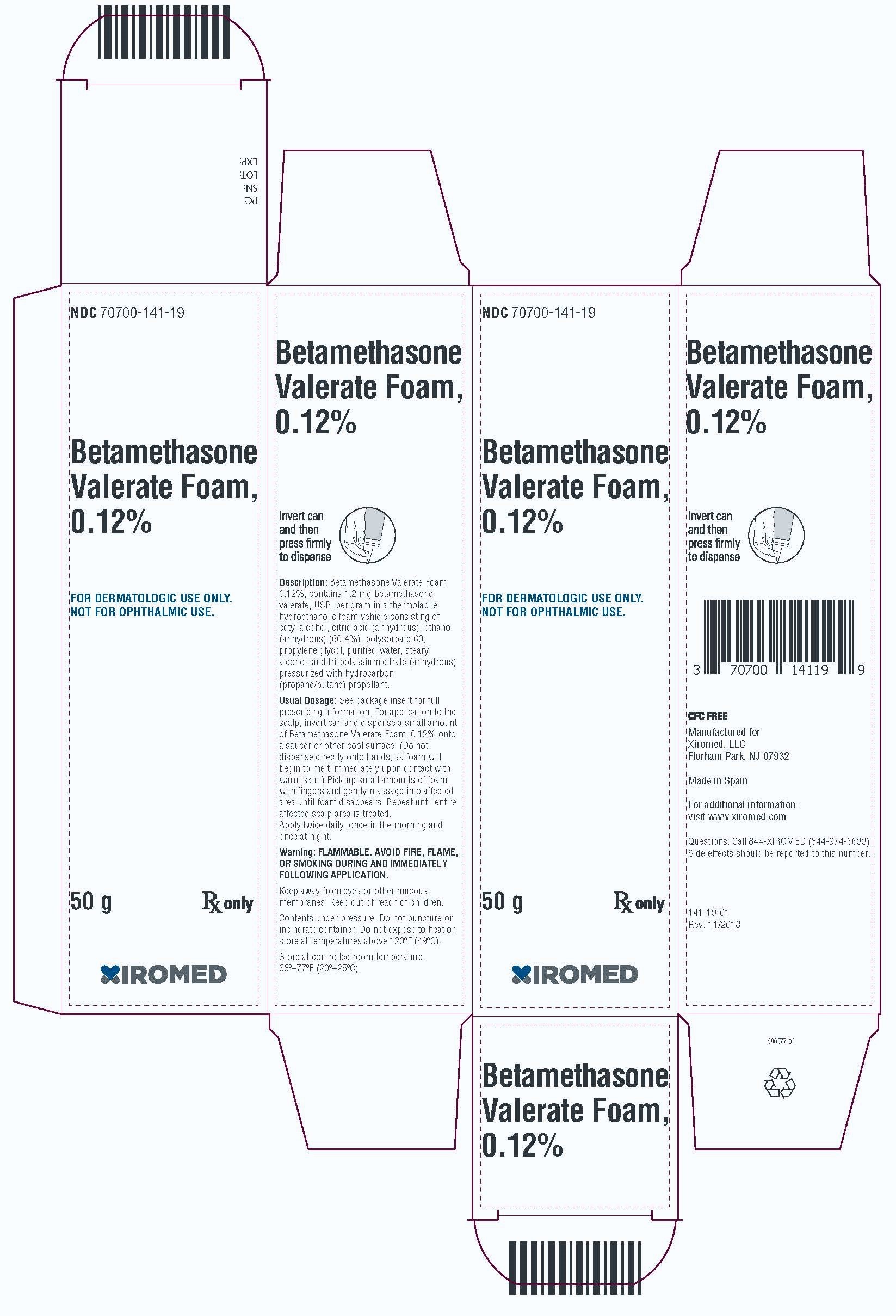
PRINCIPAL DISPLAY PANEL - 50 g CartonPrincipal Display Panel - NDC 70700-141-19 - Betamethasone Valerate Foam, 0.12% 50g - Rx Only
-
PRINCIPAL DISPLAY PANEL - 50 g LabelPrincipal Display Panel - NDC 70700-141-19 - Betamethasone Valerate Foam, 0.12% 50g - Rx Only
-
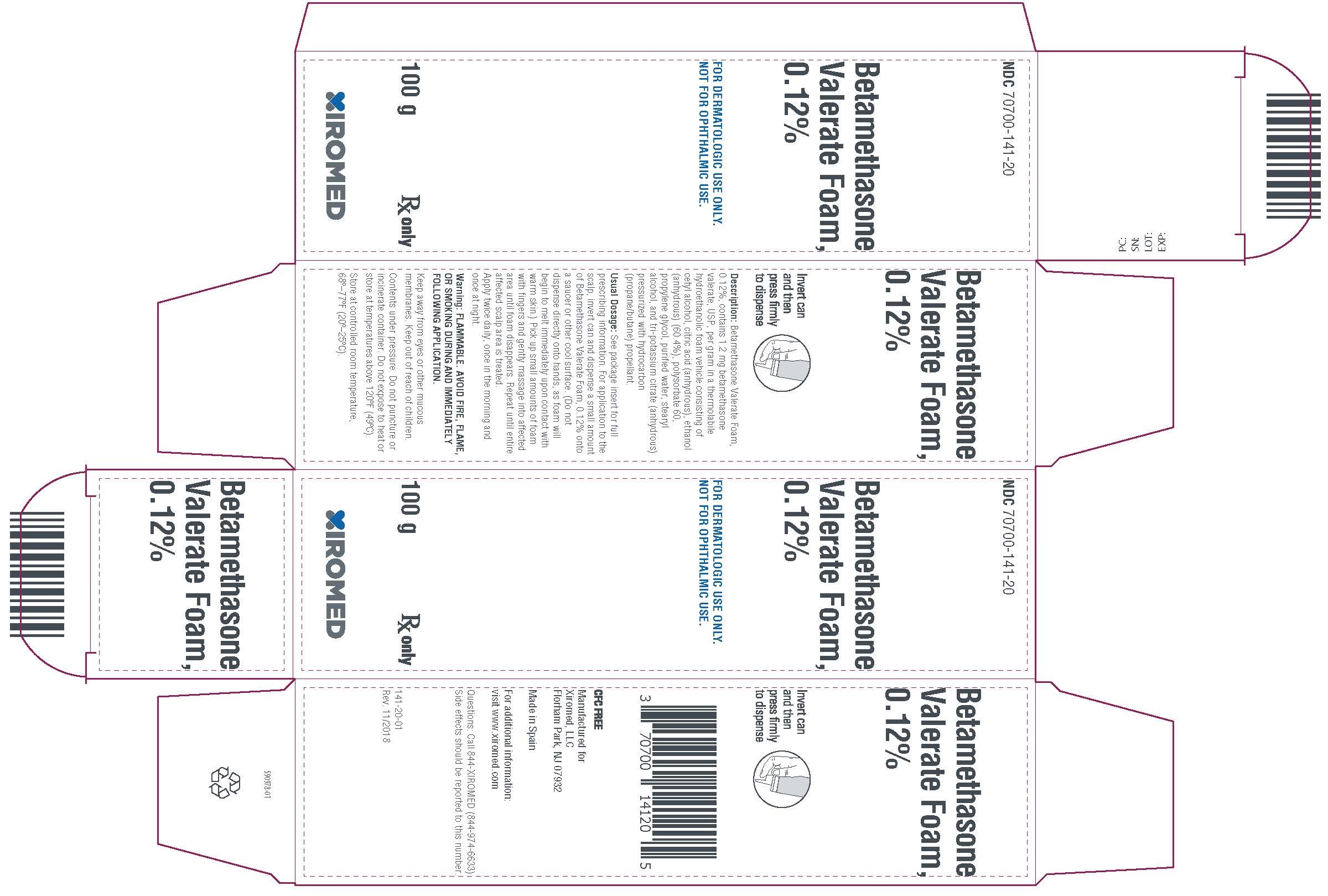
PRINCIPAL DISPLAY PANEL -100 g CartonPrincipal Display - NDC 70700-141-20 - Betamethasone Valerate Foam, 0.12% 100g - Rx Only
-
PRINCIPAL DISPLAY PANEL -100 g LabelPrincipal Display - NDC 70700-141-20 - Betamethasone Valerate Foam, 0.12% 100g - Rx Only
-
INGREDIENTS AND APPEARANCEProduct Information