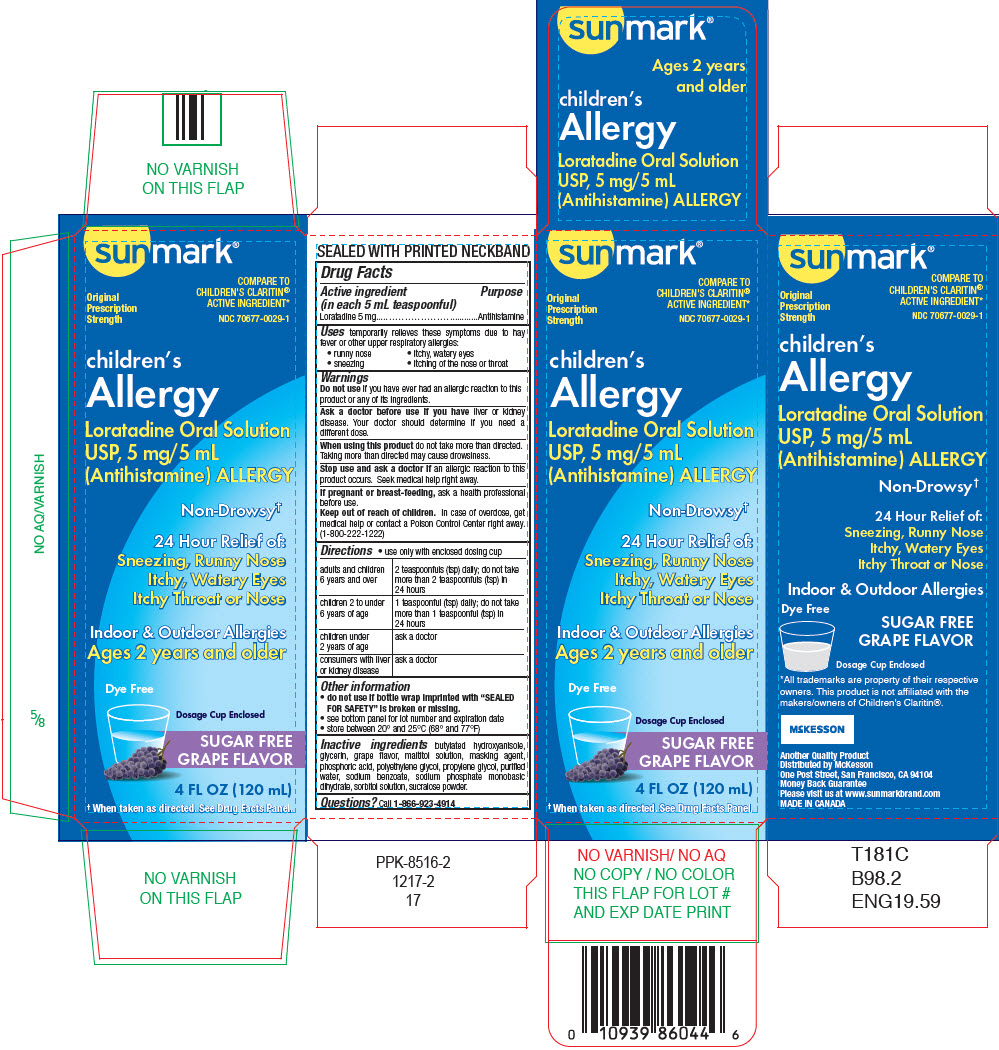
Label: SUNMARK CHILDRENS LORATADINE SRP SF GRAPE- loratadine solution
- NDC Code(s): 70677-0029-1
- Packager: Strategic Sourcing Services LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated December 19, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONDrug Facts
-
Active ingredient (in each 5 mL teaspoonful)Loratadine 5 mg
-
PurposeAntihistamine
-
Usestemporarily relieves these symptoms due to hay fever or other upper respiratory allergies: runny nose - itchy, watery eyes - sneezing - itching of the nose or throat
-
WarningsDo not useif you have ever had an allergic reaction to this product or any of its ingredients. Ask a doctor before use if you haveliver or kidney disease. Your doctor should determine ...
-
Directionsuse only with enclosed dosing cup - adults and children 6 years and over2 teaspoonfuls (tsp) daily; do not take more than 2 teaspoonfuls (tsp) in 24 hours - children 2 to under 6 years of ...
-
Other informationdo not use if bottle wrap imprinted with "SEALED FOR SAFETY" is broken or missing. see bottom panel for lot number and expiration date - store between 20° and 25°C (68° and 77°F)
-
Inactive ingredientsbutylated hydroxyanisole, glycerin, grape flavor, maltitol solution, masking agent, phosphoric acid, polyethylene glycol, propylene glycol, purified water, sodium benzoate, sodium phosphate ...
-
Questions?Call - 1-866-923-4914
-
SPL UNCLASSIFIED SECTIONDistributed by McKesson - One Post Street, San Francisco, CA 94104
-
PRINCIPAL DISPLAY PANEL - 120 mL Bottle Cartonsunmark - ® Original - Prescription - Strength - COMPARE TO - CHILDREN'S CLARITIN - ® ACTIVE INGREDIENT* NDC 70677-0029-1 - children's - Allergy - Loratadine Oral ...
-
INGREDIENTS AND APPEARANCEProduct Information