Label: KETOROLAC TROMETHAMINE solution
- NDC Code(s): 70512-790-03, 70512-790-05, 70512-790-10
- Packager: SOLA Pharmaceuticals, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated April 23, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use KETOROLAC TROMETHAMINE OPHTHALMIC SOLUTION safely and effectively. See full prescribing information for KETOROLAC TROMETHAMINE ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEKetorolac tromethamine ophthalmic solution is indicated for the temporary relief of ocular itching due to seasonal allergic conjunctivitis. Ketorolac tromethamine ophthalmic solution is also ...
-
2 DOSAGE AND ADMINISTRATION2.1 Recommended Dosing - Patient Dosing - The recommended dose of ketorolac tromethamine ophthalmic solution is one drop four times a day to the affected eye(s) for relief of ocular itching due ...
-
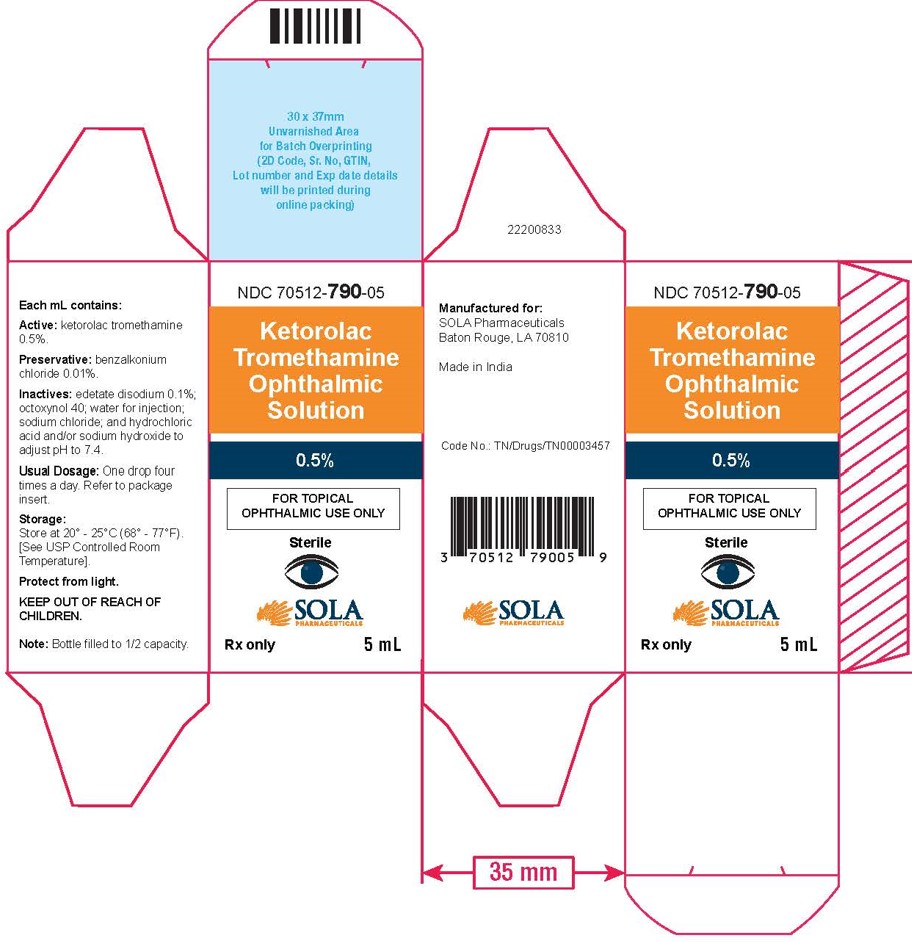
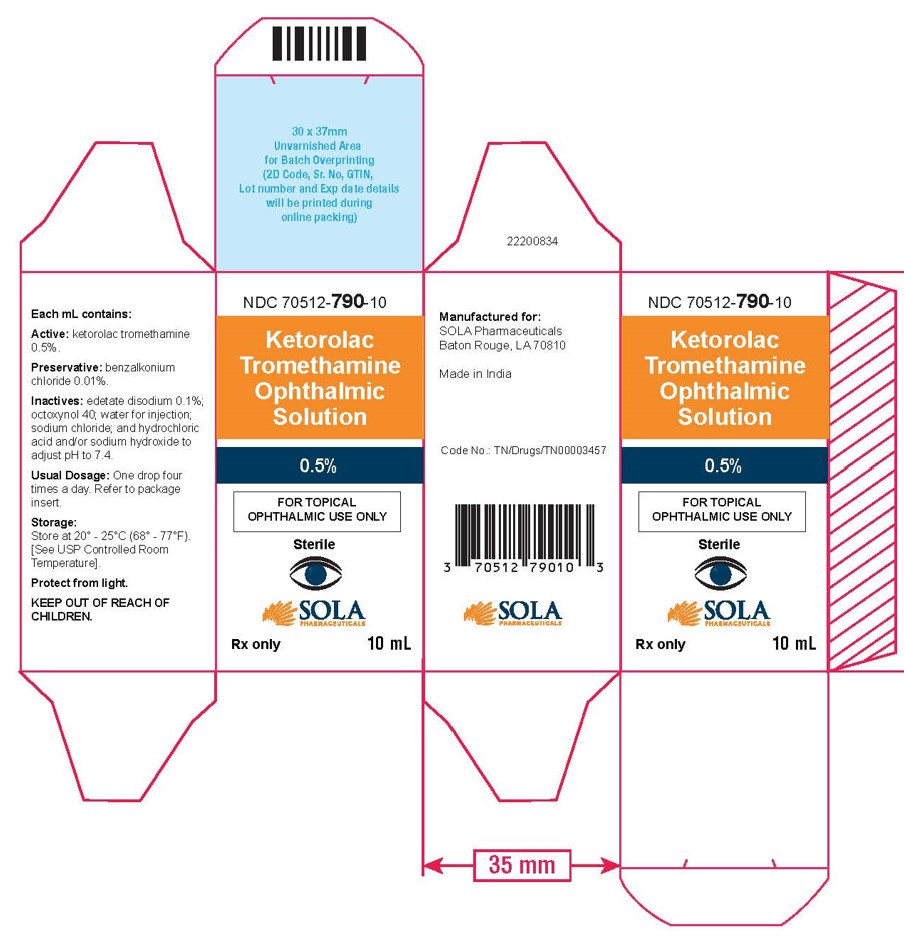
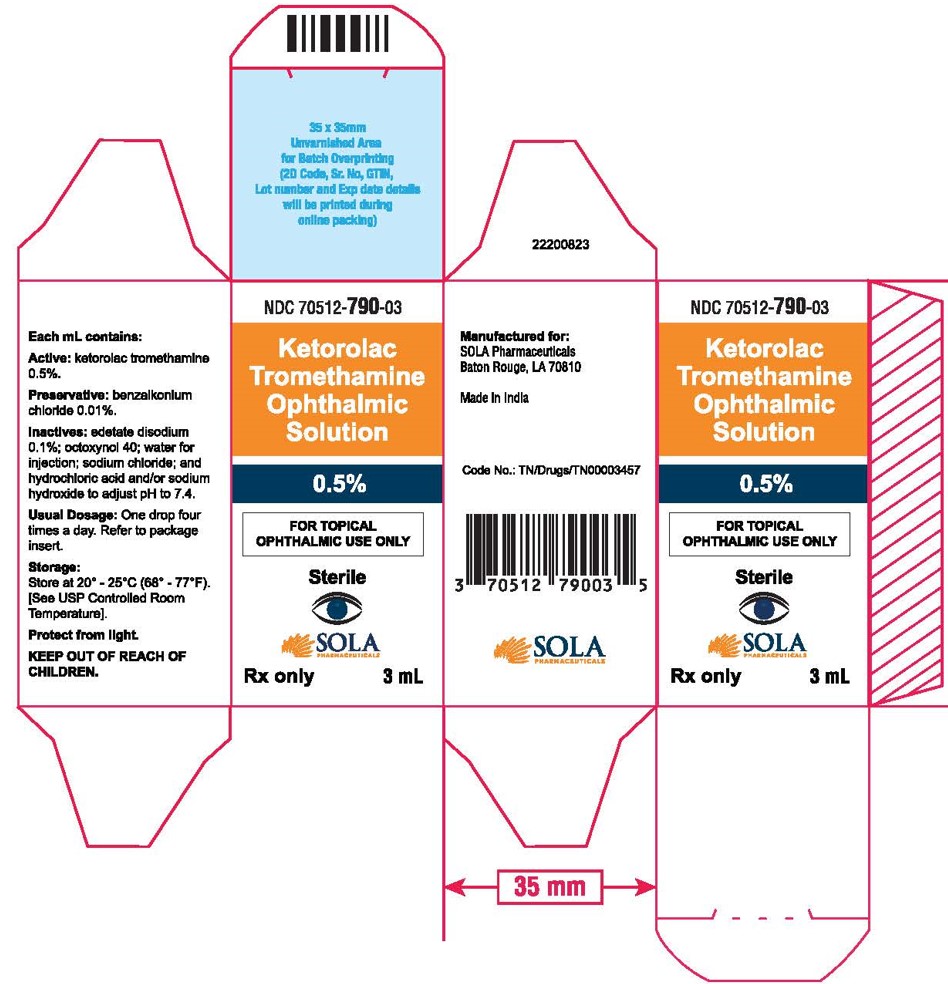
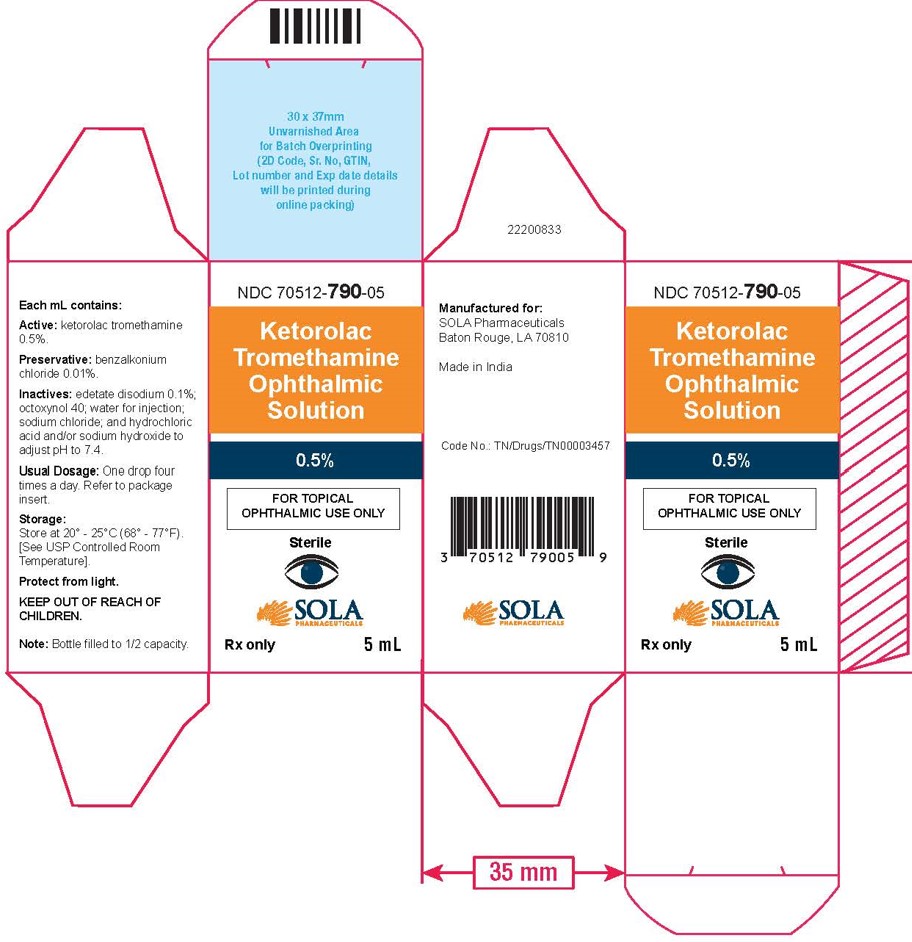
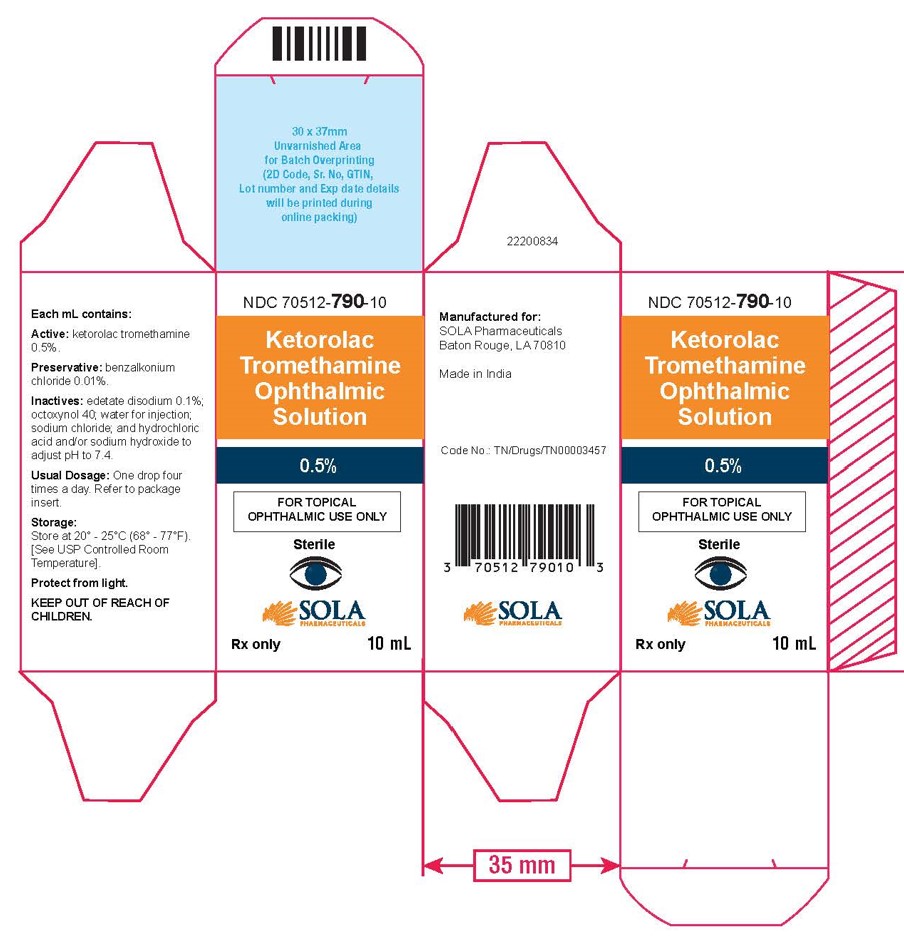
3 DOSAGE FORMS AND STRENGTHS5 mL size bottle filled with 3 mL of ketorolac tromethamine ophthalmic solution, 0.5% (5 mg/mL) 10 mL size bottle filled with 5 mL of ketorolac tromethamine ophthalmic solution, 0.5% (5 ...
-
4 CONTRAINDICATIONSKetorolac tromethamine ophthalmic solution is contraindicated in patients with previously demonstrated hypersensitivity to any of the ingredients in the formulation.
-
5 WARNINGS AND PRECAUTIONS5.1 Delayed Healing - Topical nonsteroidal anti-inflammatory drugs (NSAIDs) may slow or delay healing. Topical corticosteroids are also known to slow or delay healing. Concomitant use of topical ...
-
6 ADVERSE REACTIONS6.1 Clinical Studies Experience - Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a drug cannot be directly ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Teratogenic Effects. Pregnancy Category C - Pregnancy Category C: Ketorolac tromethamine, administered during organogenesis, was not teratogenic in rabbits and rats at oral ...
-
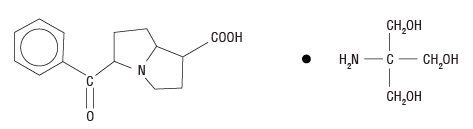
11 DESCRIPTIONKetorolac tromethamine ophthalmic solution 0.5% is a member of the pyrrolo-pyrrole group of nonsteroidal anti-inflammatory drugs (NSAIDs) for ophthalmic use. Its chemical name is (±)-5-Benzoyl-2 ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Ketorolac tromethamine is a nonsteroidal anti-inflammatory drug which, when administered systemically, has demonstrated analgesic, anti-inflammatory, and anti-pyretic ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Ketorolac tromethamine was not carcinogenic in either rats given up to 5 mg/kg/day orally for 24 months or in mice given 2 mg/kg/day ...
-
14 CLINICAL STUDIESTwo controlled clinical studies showed that ketorolac tromethamine ophthalmic solution was significantly more effective than its vehicle in relieving ocular itching caused by seasonal allergic ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGKetorolac tromethamine ophthalmic solution 0.5% is supplied sterile, in white opaque plastic LDPE bottles with white droppers, with gray high density polyethylene (HDPE) caps as follows: 3mL in 5 ...
-
17 PATIENT COUNSELING INFORMATION17.1 Slow or Delayed Healing - Patients should be informed of the possibility that slow or delayed healing may occur while using nonsteroidal anti-inflammatory drugs (NSAIDs). 17.2 Avoiding ...
-
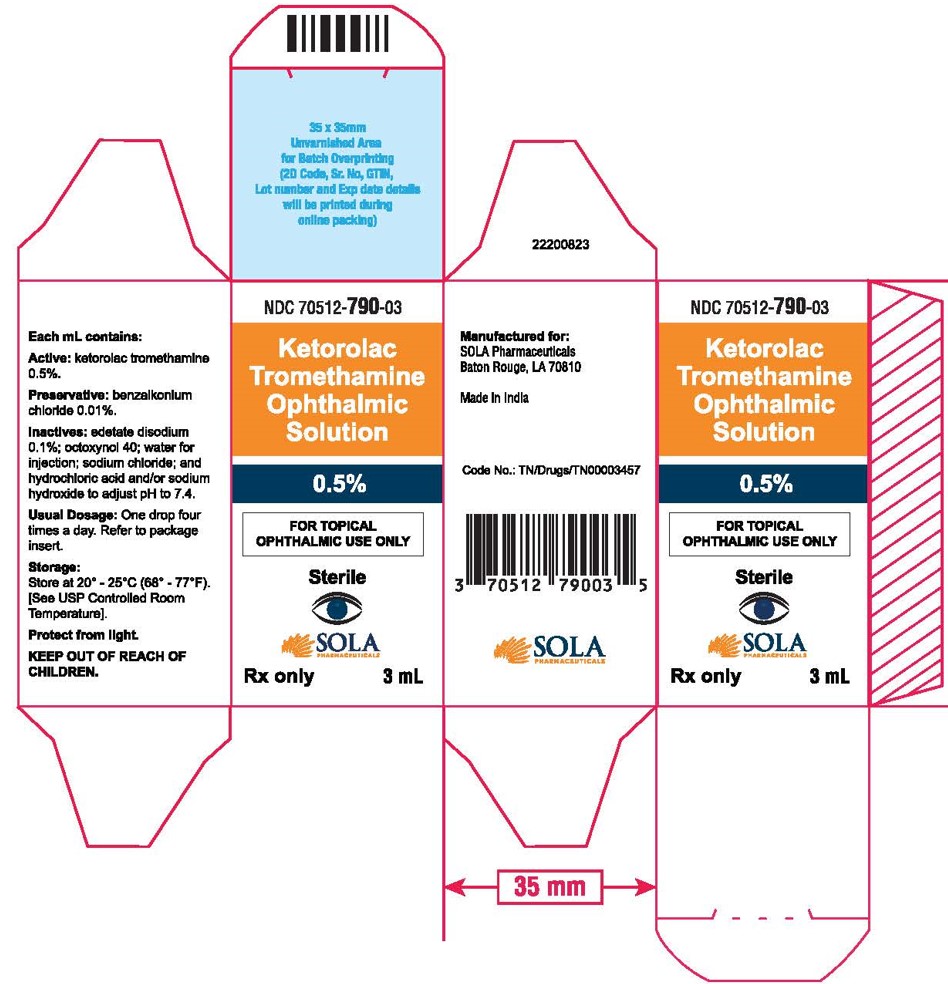
PRINCIPAL DISPLAY PANELPrincipal Display Panel Text for 3mL Container Label: NDC 70512-790-03 - Ketrolac Tromethamine - Ophthalmic Solution - 0.5% FOR TOPICAL OPHTHALMIC USE ONLY - Sterile - Rx ...
-
PRINCIPAL DISPLAY PANELPrincipal Display Panel Text for 3mL Carton Label: NDC 70512-790-03 - Ketorolac - Tromethamine - Opthalmic Solution - 0.5% FOR TOPICAL - OPHTHALMIC USE ONLY ...
-
INGREDIENTS AND APPEARANCEProduct Information