Label: SUMATRIPTAN injection, solution
- NDC Code(s): 70069-804-01, 70069-804-05
- Packager: Somerset Therapeutics, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated September 22, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use SUMATRIPTAN INJECTION safely and effectively. See full prescribing information for SUMATRIPTAN INJECTION. SUMATRIPTAN injection ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGESumatriptan Injection, USP is indicated in adults for (1) the acute treatment of migraine, with or without aura, and (2) the acute treatment of cluster headache. Limitations of Use: • Use ...
-
2 DOSAGE AND ADMINISTRATION2.1 Dosing Information - The maximum single recommended adult dose of Sumatriptan injection for the acute treatment of migraine or cluster headache is 6 mg injected subcutaneously. For the ...
-
3 DOSAGE FORMS AND STRENGTHSInjection: 6 mg single-dose vial. Each 0.5 mL injection contains 8.4 mg of sumatriptan succinate equivalent to 6 mg of sumatriptan.
-
4 CONTRAINDICATIONSSumatriptan Injection is contraindicated in patients with: • Ischemic coronary artery disease (CAD) (angina pectoris, history of myocardial infarction, or documented silent ischemia) or ...
-
5 WARNINGS AND PRECAUTIONS5.1 Myocardial Ischemia, Myocardial Infarction, and Prinzmetal's Angina - The use of sumatriptan injection is contraindicated in patients with ischemic or vasospastic CAD. There have been rare ...
-
6 ADVERSE REACTIONSThe following serious adverse reactions are described below and elsewhere in the labeling: · Myocardial ischemia, myocardial infarction, and Prinzmetal’s angina [see Warnings and ...
-
7 DRUG INTERACTIONS7.1 Ergot-Containing Drugs - Ergot-containing drugs have been reported to cause prolonged vasospastic reactions. Because these effects may be additive, use of ergotamine-containing or ergot-type ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Data from a prospective pregnancy exposure registry and epidemiological studies of pregnant women have not detected an increased frequency of birth defects or a ...
-
10 OVERDOSAGECoronary vasospasm was observed after intravenous administration of sumatriptan injection [see Contraindications (4)]. Overdoses would be expected from animal data (dogs at 0.1 g/kg, rats at 2 ...
-
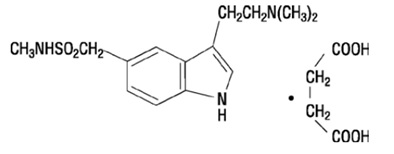
11 DESCRIPTIONSumatriptan injection, USP contains sumatriptan succinate, a selective 5-HT1B/1D receptor agonist. Sumatriptan succinate is chemically designated as ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Sumatriptan binds with high affinity to human cloned 5-HT1B/1D receptors. Sumatriptan presumably exerts its therapeutic effects in the treatment of migraine and cluster ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - In carcinogenicity studies in mouse and rat, sumatriptan was administered orally for 78 weeks and 104 weeks ...
-
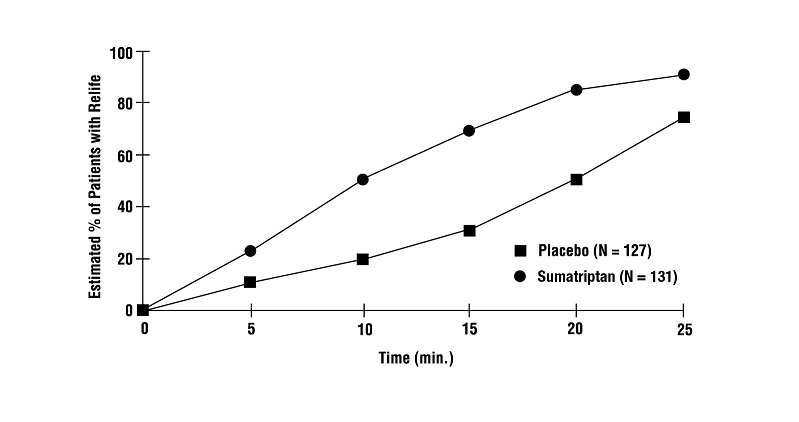
14 CLINICAL STUDIES14.1 Migraine - In controlled clinical trials enrolling more than 1,000 patients during migraine attacks who were experiencing moderate or severe pain and 1 or more of the symptoms enumerated ...
-
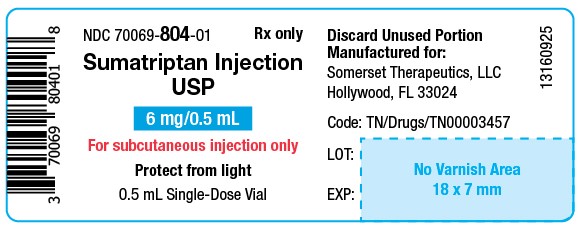
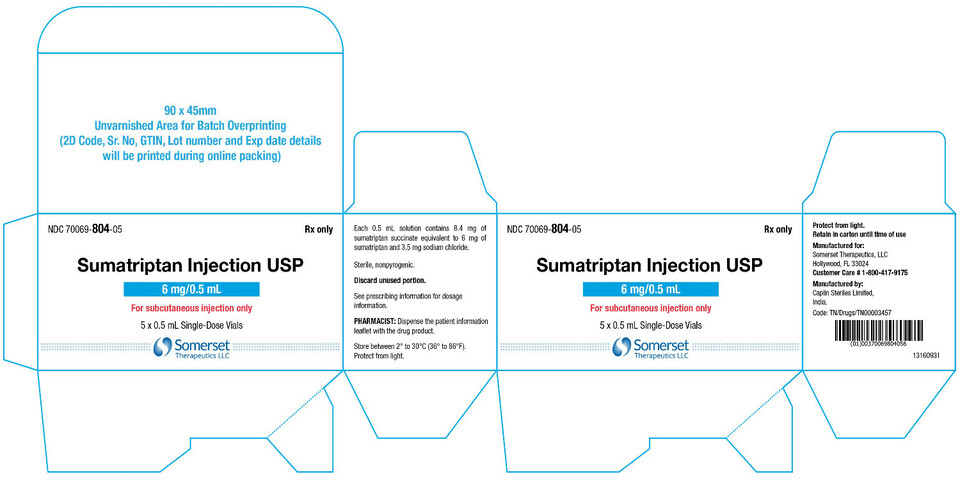
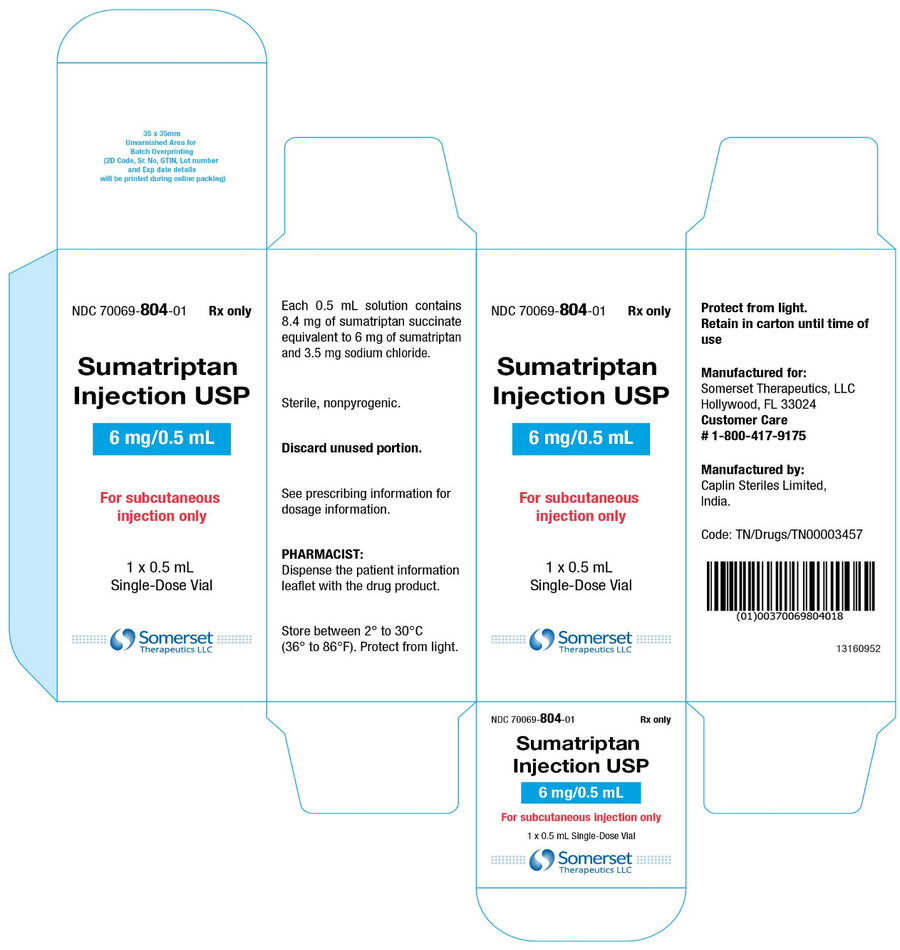
16 HOW SUPPLIED/STORAGE AND HANDLINGSumatriptan injection, USP contains sumatriptan (base) as the succinate salt and is supplied as a clear, colorless to pale yellow, sterile, nonpyrogenic solution as follows: Sumatriptan injection ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use). Risk of Myocardial Ischemia and/or Infarction, Prinzmetal’s Angina, Other ...
-
PATIENT INFORMATIONSumatriptan (soo’’ ma trip’ tan) Injection USP - What is the most important information I should know about sumatriptan? Sumatriptan can cause serious side effects, including: Heart ...
-
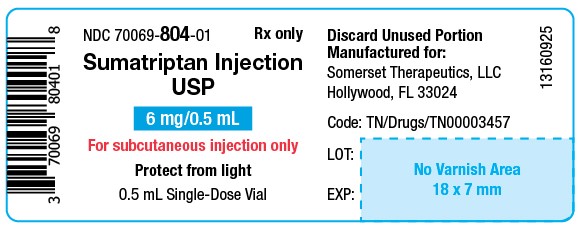
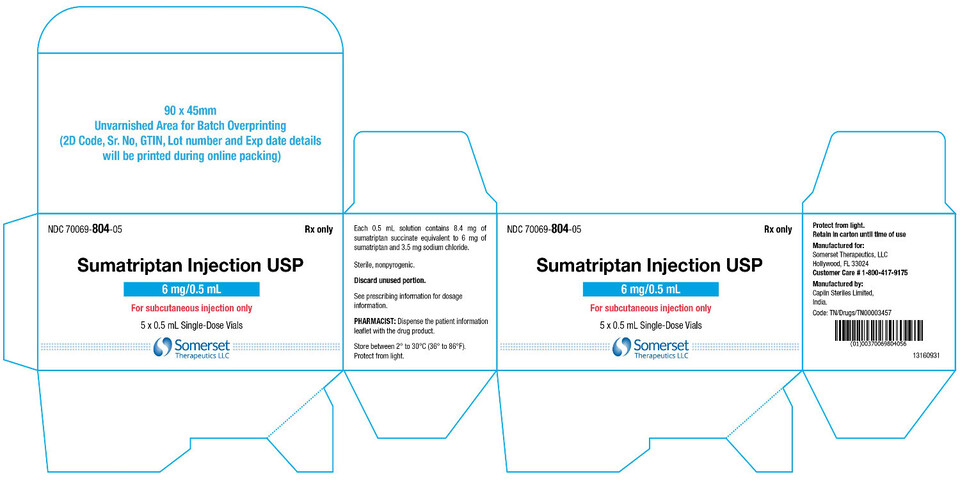
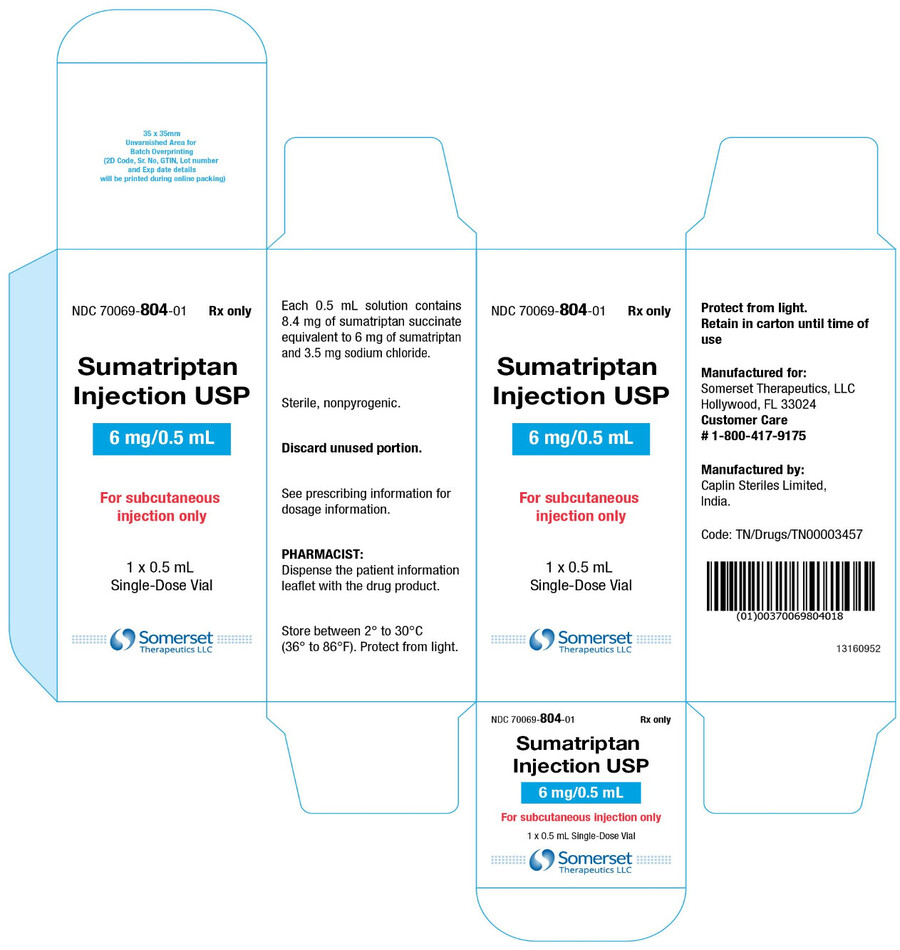
PACKAGE LABEL.PRINCIPAL DISPLAY PANELSumatriptan Injection USP, 6 mg/0.5 mL - Vial Label - NDC 70069- 804-01 - Rx Only - Sumatriptan Injection USP - 6 mg/0.5 mL - For subcutaneous injection only - Protect from light - 0.5 mL ...
-
INGREDIENTS AND APPEARANCEProduct Information