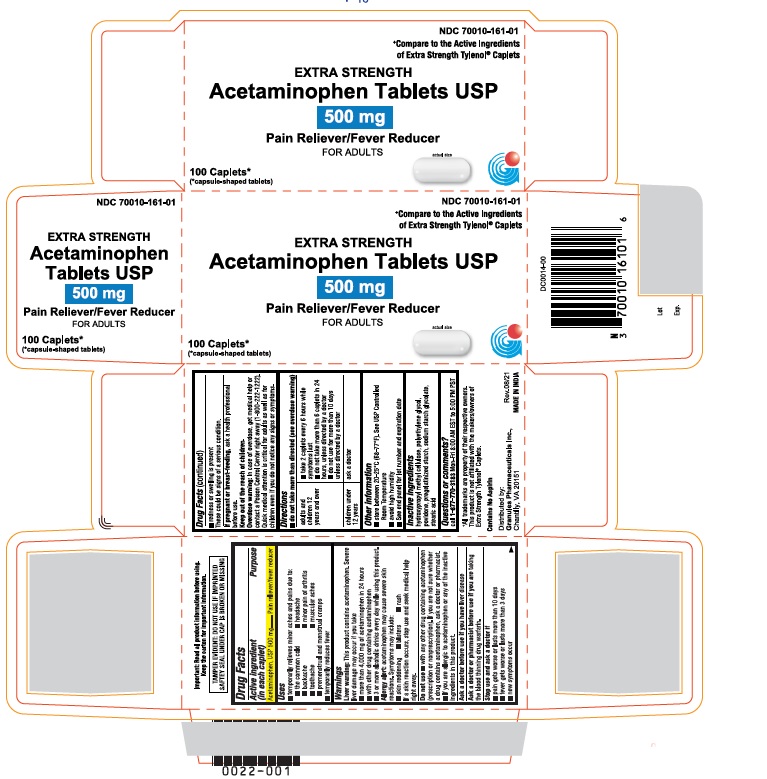
Label: ACETAMINOPHEN tablet
- NDC Code(s): 70010-161-01, 70010-161-05, 70010-161-10
- Packager: Granules Pharmaceuticals Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated April 2, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
ACTIVE INGREDIENT (IN EACH CAPLET)Acetaminophen, USP 500 mg
-
PURPOSEPain reliever/fever reducer
-
USES• temporarily relieves minor aches and pains due to: • the common cold - • headache - • backache - • minor pain of arthritis - • toothache - • muscular aches - • premenstrual ...
-
WARNINGSLiver warning: This product contains acetaminophen. Severe Liver damage may occur if you take - • more than 4,000 mg of acetaminophen 24 hours. • with other drugs containing ...
-
Do not use• with any other drugs containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist. • if you are ...
-
Ask a doctor before use if you haveliver disease.
-
Ask a doctor or pharmacist before use if you are taking the blood thinning drug warfarin.
-
Stop use and ask doctor if• pain gets worse or lasts more than 10 days - • fever gets worse or lasts more than 3 days - • new symptoms occur - • redness or swelling is present - These could be signs ...
-
If pregnant or breast-feedingask a health professional before use.
-
Keep out of reach of childrenOver dose warning: In case of overdose, get medical help or Contact Poison Control Center right away. (1-800-222-1222) Quick medical attention is critical for adults as well as for children even ...
-
DIRECTIONS• do not take more than directed (see overdose warning) adults and children - 12 years and over - • take 2 caplets every 6 hours while symptoms last - • do not take ...
-
OTHER INFORMATION• store at 20-25°C (68-77°F). See USP Controlled Room Temparature - • avoid high humidity - • See end panel for lot number and expiration date
-
INACTIVE INGREDIENTShydroxyethyl methyl cellulose, polyethylene glycol, povidone, pregelatinized starch, sodium starch glycolate, stearic acid.
-
QUESTIONS OR COMMENTS ?Contact - 1-877-770-3183 Mon-Fri 8:00 AM EST to 5:00 PM PST. +All trademarkes are property of their reapective owners. This product is not affliated with the makers/owners of - Extra Strength ...
-
PRINCIPAL DISPLAY PANEL
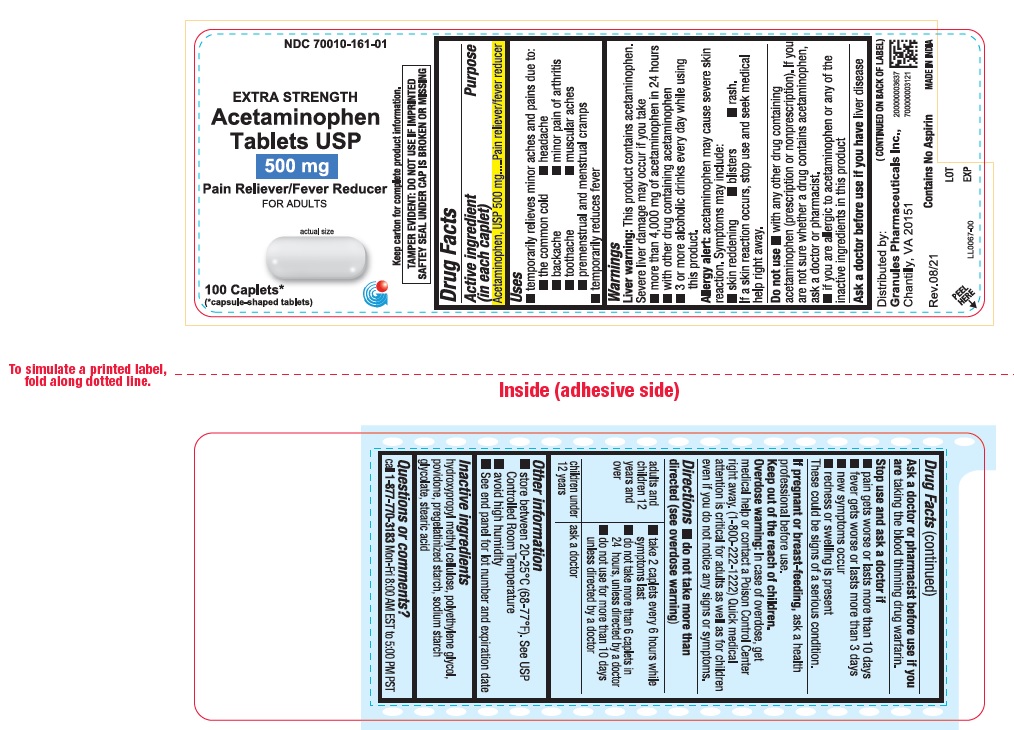 ...
... -
INGREDIENTS AND APPEARANCEProduct Information