Label: ALLOPURINOL tablet
-
Contains inactivated NDC Code(s)
NDC Code(s): 69967-008-01, 69967-008-02, 69967-008-03, 69967-009-01, view more - Packager: Arise Pharamaceuticals LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated December 9, 2019
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
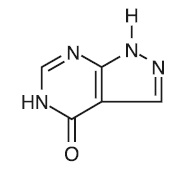
DESCRIPTIONAllopurinol has the following structural formula: Allopurinol is known chemically as 1,5-dihydro-4H-pyrazolo [3,4-d]pyrimidin-4-one. It is a xanthine oxidase inhibitor which is ...
-
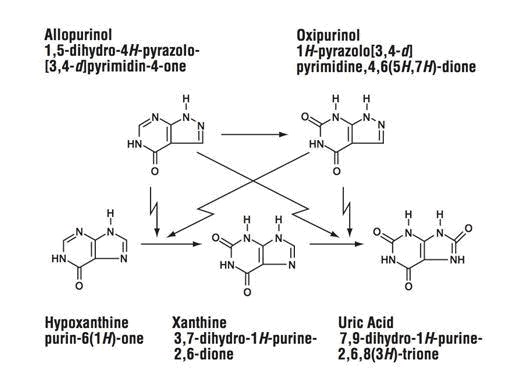
CLINICAL PHARMACOLOGYAllopurinol acts on purine catabolism, without disrupting the biosynthesis of purines. It reduces the production of uric acid by inhibiting the biochemical reactions immediately preceding its ...
-
INDICATIONS AND USAGETHIS IS NOT AN INNOCUOUS DRUG. IT IS NOT RECOMMENDED FOR THE TREATMENT OF ASYMPTOMATIC HYPERURICEMIA. Allopurinol tablets reduce serum and urinary uric acid concentrations. Its use should be ...
-
CONTRAINDICATIONSPatients who have developed a severe reaction to allopurinol tablets should not be restarted on the drug.
-
WARNINGSALLOPURINOL TABLETS SHOULD BE DISCONTINUED AT THE FIRST APPEARANCE OF SKIN RASH OR OTHER SIGNS WHICH MAY INDICATE AN ALLERGIC REACTION. In some instances a skin rash may be followed by more ...
-
PRECAUTIONSGeneral - An increase in acute attacks of gout has been reported during the early stages of administration of allopurinol tablets, even when normal or subnormal serum uric acid levels have been ...
-
ADVERSE REACTIONSData upon which the following estimates of incidence of adverse reactions are made are derived from experiences reported in the literature, unpublished clinical trials and voluntary reports ...
-
OVERDOSAGEMassive overdosing or acute poisoning by allopurinol tablets has not been reported. In mice, the 50% lethal dose (LD50) is 160 mg/kg given intraperitoneally (IP) with deaths delayed up to 5 ...
-
DOSAGE & ADMINISTRATIONThe dosage of allopurinol tablets to accomplish full control of gout and to lower serum uric acid to normal or near-normal levels varies with the severity of the disease. The average is 200 to ...
-
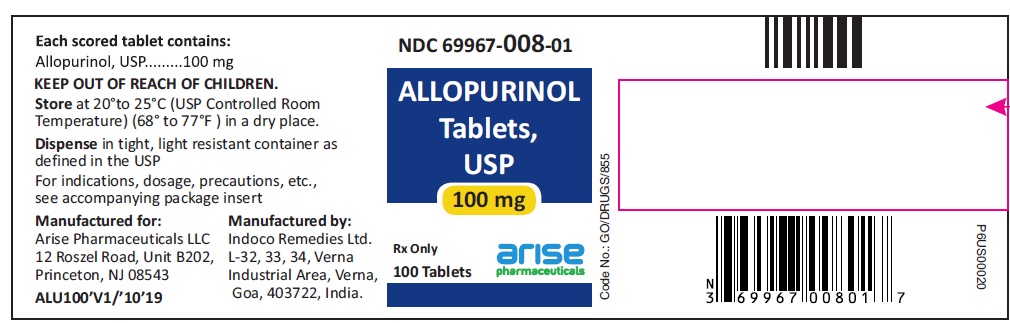
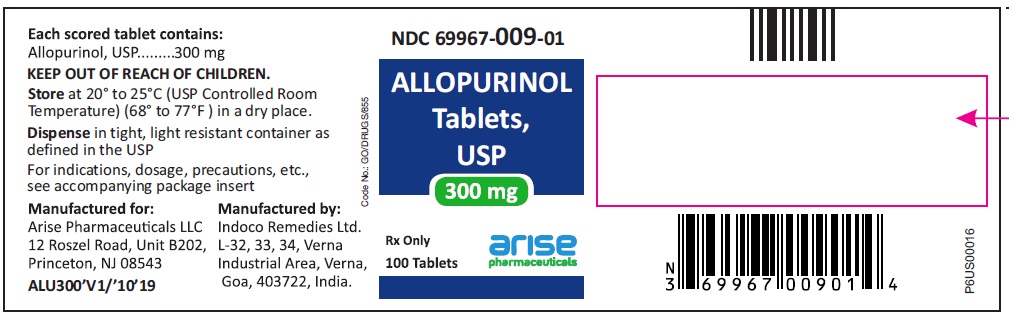
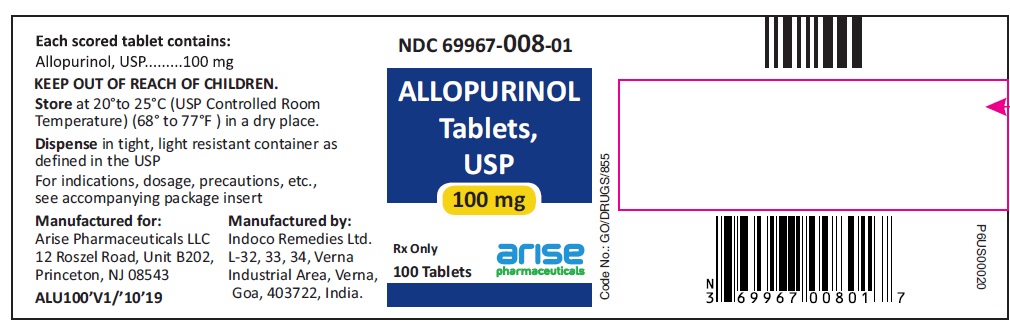
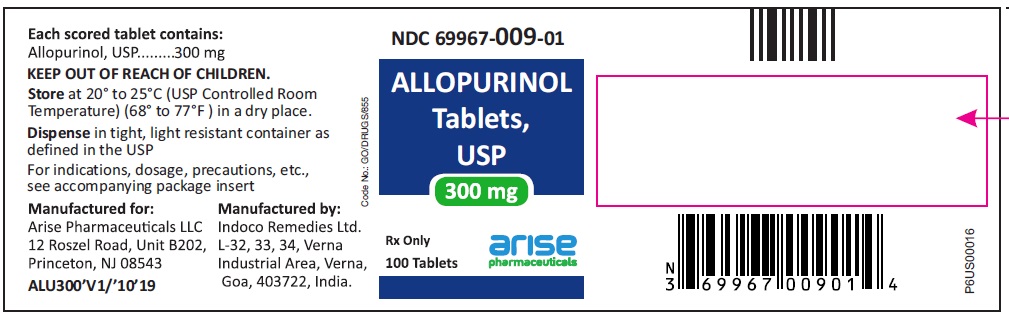
HOW SUPPLIED100-mg (white to off white) scored, flat cylindrical tablets with "I" and "135" on either side of the break line on one side and plain on other side, bottles of 100 (NDC 69967-008-01), 500 (NDC ...
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL100 mg 100 Tablets Bottle - 100 Tablets NDC 69967-008-01 - Allopurinol Tablets USP - Each scored tablet contains - 100 mg - Rx Only - 100 mg 1000 Tablets Bottle - 1000 ...
-
INGREDIENTS AND APPEARANCEProduct Information