Label: DESMOPRESSIN ACETATE tablet
- NDC Code(s): 69918-101-01, 69918-201-01
- Packager: Nordic Pharma, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated December 6, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
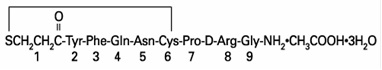
DESCRIPTIONDesmopressin Acetate Tablets (desmopressin acetate) are a synthetic analogue of the natural pituitary hormone 8-arginine vasopressin (ADH), an antidiuretic hormone affecting renal water ...
-
CLINICAL PHARMACOLOGYDesmopressin Acetate Tablets contain as active substance, desmopressin acetate, a synthetic analogue of the natural hormone arginine vasopressin. Central Diabetes Insipidus: Dose response ...
-
INDICATIONS AND USAGECentral Diabetes Insipidus: Desmopressin Acetate Tablets are indicated as antidiuretic replacement therapy in the management of central diabetes insipidus and for the management of the ...
-
CONTRAINDICATIONSDesmopressin Acetate Tablets are contraindicated in individuals with known hypersensitivity to desmopressin acetate or to any of the components of Desmopressin Acetate Tablets. Desmopressin ...
-
WARNINGS1. Very rare cases of hyponatremia have been reported from world-wide postmarketing experience in patients treated with desmopressin acetate. Desmopressin acetate is a potent antidiuretic which ...
-
PRECAUTIONSGeneral: Intranasal formulations of desmopressin acetate at high doses and Desmopressin Acetate Injection have infrequently produced a slight elevation of blood pressure which disappears with ...
-
ADVERSE REACTIONSInfrequently, large doses of the intranasal formulations of desmopressin acetate and Desmopressin Acetate Injection have produced transient headache, nausea, flushing and mild abdominal cramps ...
-
OVERDOSAGESigns of overdose may include confusion, drowsiness, continuing headache, problems with passing urine and rapid weight gain due to fluid retention. (See WARNINGS.) In case of overdose, the dose ...
-
DOSAGE AND ADMINISTRATIONCentral Diabetes Insipidus: The dosage of Desmopressin Acetate Tablets must be determined for each individual patient and adjusted according to the diurnal pattern of response. Response ...
-
HOW SUPPLIEDStrengthSizeNDC codeColorMarkings - 0.1 mgBottle of 100NDC 69918-101-01White - 0.2 mgBottle of 100NDC 69918-201-01White - Store at Controlled Room Temperature 20 to 25°C (68 to 77°F ...
-
SPL UNCLASSIFIED SECTIONManufactured for: Nordic Pharma, Inc. Berwyn, PA 19312 - www.nordicpharmausa.com - The Nordic Pharma Logo is a trademark of Nordic Group B.V. Origin Sweden - Rev. 10/2024 - 2009056650
-
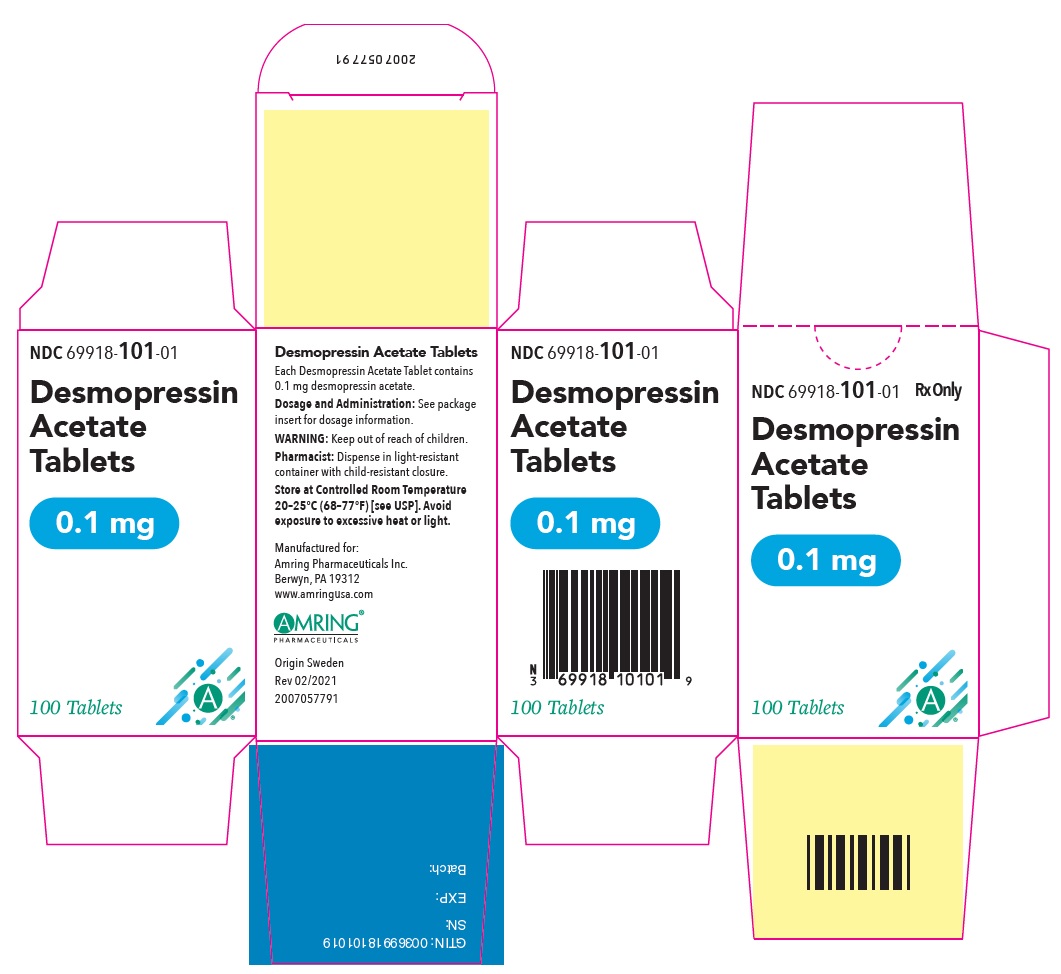
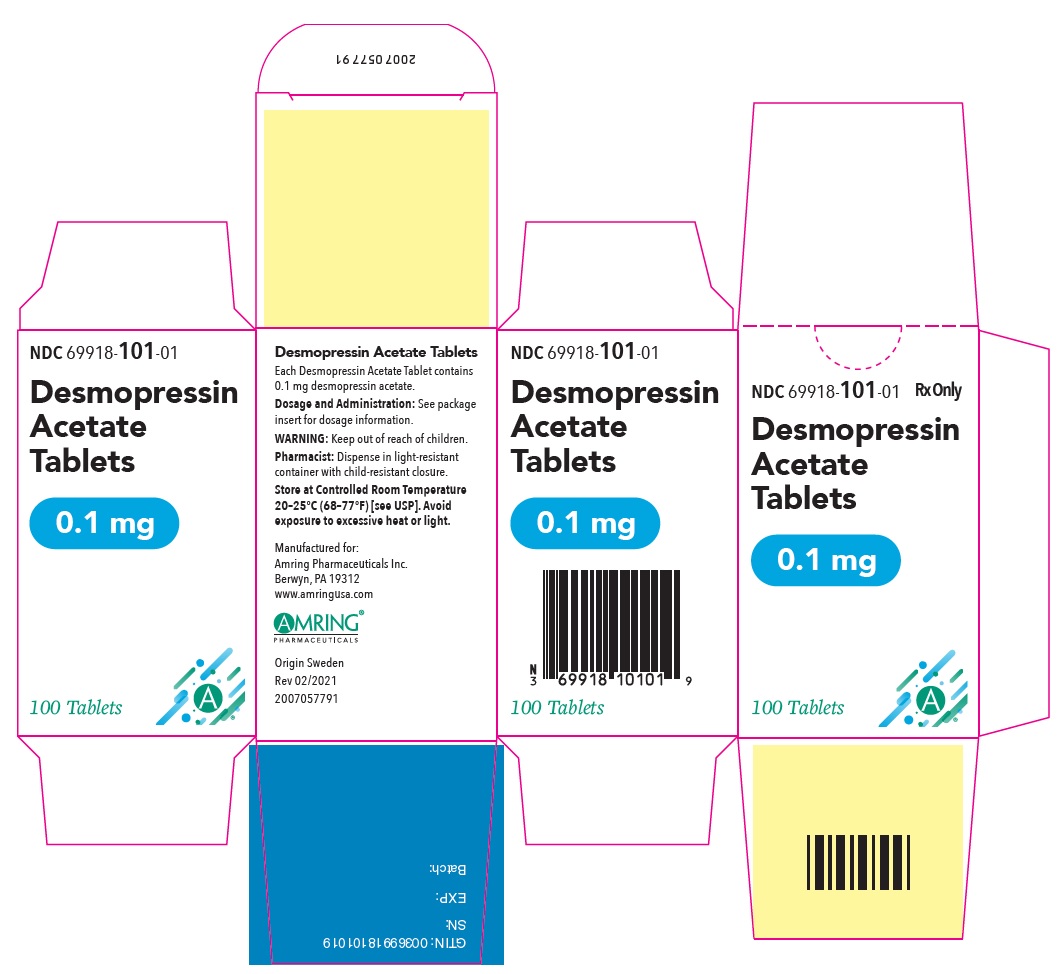
PRINCIPAL DISPLAY PANEL - 0.1 mg Tablet Bottle CartonNDC 69918-101-01 - Rx Only - Desmopressin - Acetate - Tablets - 0.1 mg - 100 Tablets
-
PRINCIPAL DISPLAY PANEL - 0.1 mg TabletNDC 69918-101-01 - Rx Only - Desmopressin - Acetate Tablets - 0.1 mg - 100 Tablets
-
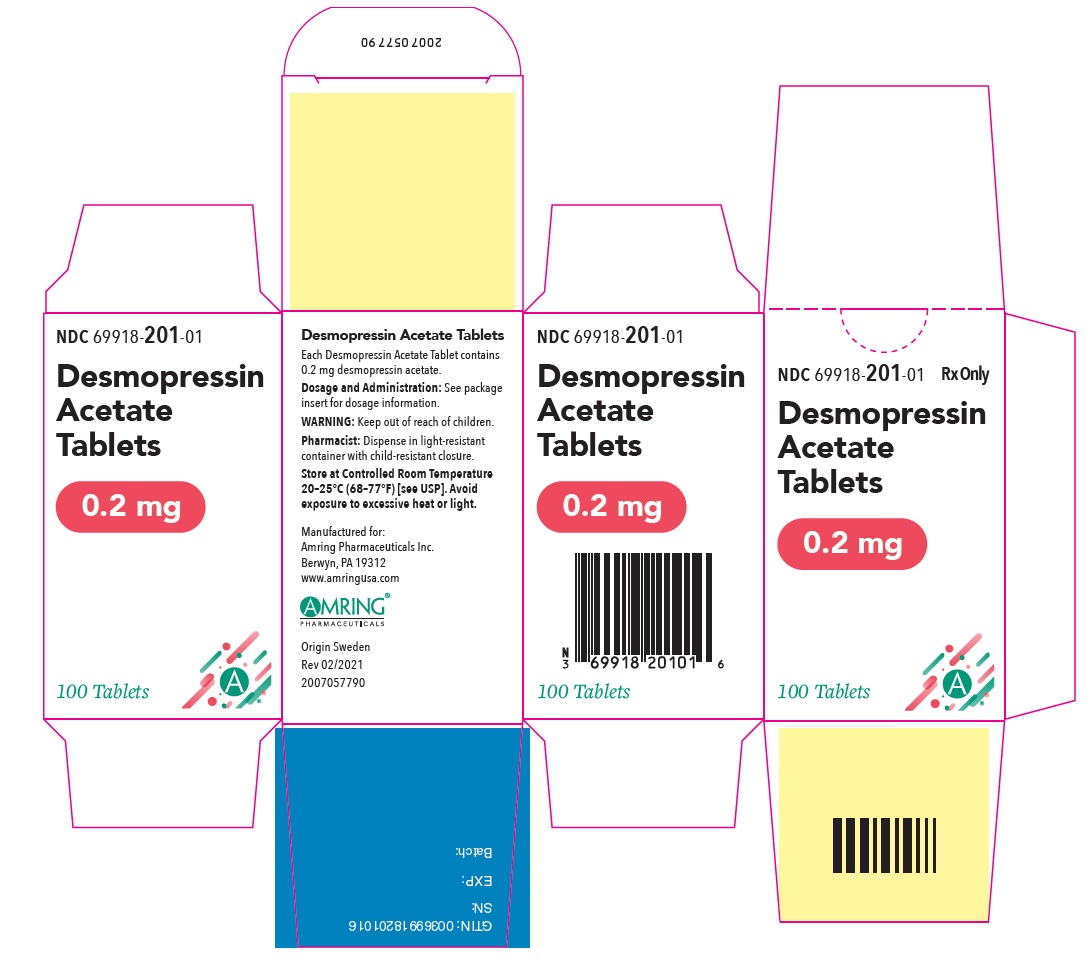
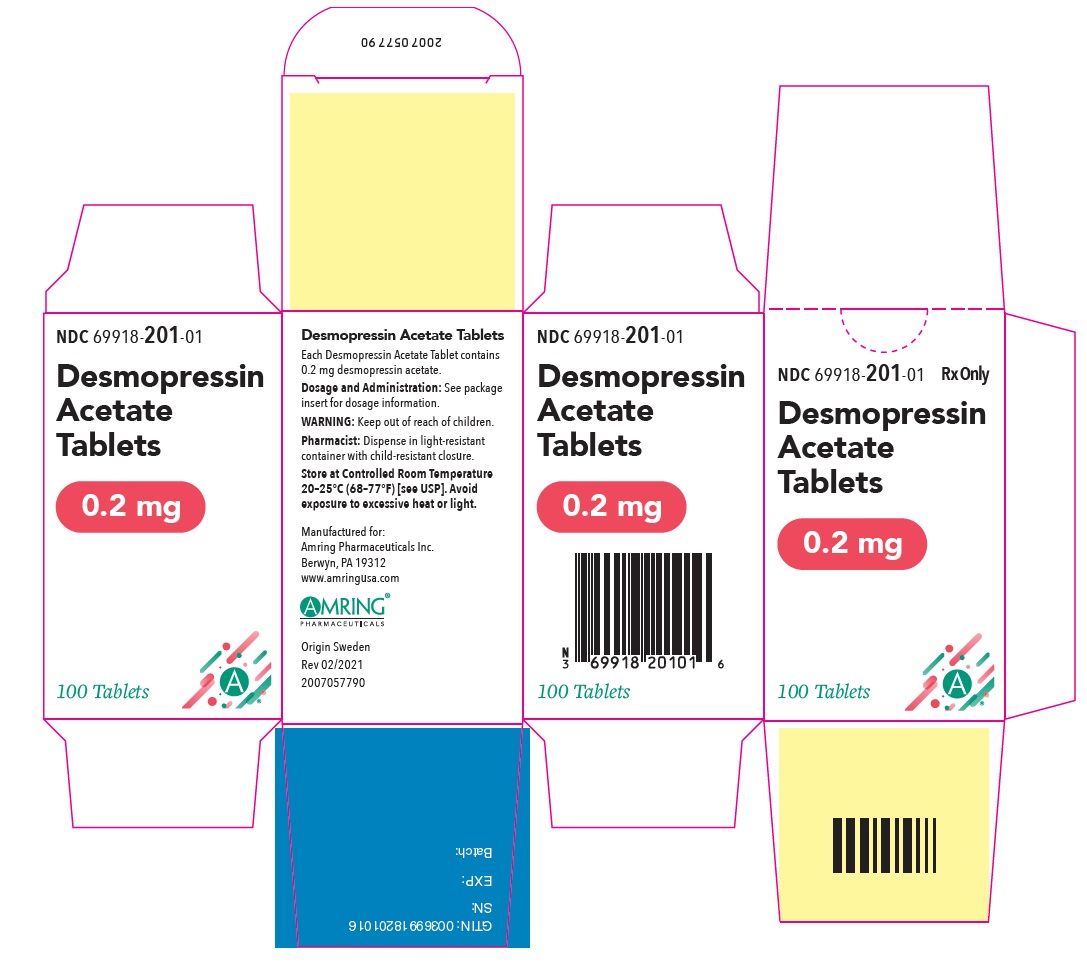
PRINCIPAL DISPLAY PANEL - 0.2 mg Tablet Bottle CartonNDC 69918-201-01 - Rx Only - Desmopressin - Acetate - Tablets - 0.2 mg - 100 Tablets
-
PRINCIPAL DISPLAY PANEL - 0.2 mg TabletNDC 69918-201-01 - Rx Only - Desmopressin - Acetate Tablets - 0.2 mg - 100 Tablets
-
INGREDIENTS AND APPEARANCEProduct Information