Label: CYANOCOBALAMIN injection, solution
- NDC Code(s): 69680-112-01, 69680-112-10, 69680-112-25, 69680-113-10, view more
- Packager: Vitruvias Therapeutics
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated December 20, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONRx Only
-
DESCRIPTIONCyanocobalamin Injection, USP is a sterile solution of cyanocobalamin for intramuscular or subcutaneous injection. Each mL contains 1000 mcg cyanocobalamin. Each vial also contains Sodium ...
-
CLINICAL PHARMACOLOGYVitamin B12 is essential to growth, cell reproduction, hematopoiesis, and nucleoprotein and myelin synthesis. Cyanocobalamin is quantitatively and rapidly absorbed from intramuscular and ...
-
INDICATIONS AND USAGECyanocobalamin is indicated for vitamin B12 deficiencies due to malabsorption which may be associated with the following conditions: Addisonian (pernicious) anemia - Gastrointestinal pathology ...
-
CONTRAINDICATIONSSensitivity to cobalt and/or vitamin B12 is a contraindication.
-
WARNINGSPatients with early Leber's disease (hereditary optic nerve atrophy) who were treated with cyanocobalamin suffered severe and swift optic atrophy. Hypokalemia and sudden death may occur in severe ...
-
PRECAUTIONSGeneral Precautions - Vitamin B12 deficiency that is allowed to progress for longer than 3 months may produce permanent degenerative lesions of the spinal cord. Doses of folic acid greater than ...
-
ADVERSE REACTIONSGeneralized: Anaphylactic shock and death have been reported with administration of parenteral vitamin B12 (see WARNINGS). Cardiovascular: Pulmonary edema and congestive heart failure early in ...
-
OVERDOSAGENo overdosage has been reported with this drug.
-
DOSAGE AND ADMINISTRATIONAvoid using the intravenous route. Use of this product intravenously will result in almost all of the vitamin being lost in the urine. Pernicious Anemia - Parenteral vitamin B12 is the ...
-
HOW SUPPLIEDCyanocobalamin Injection, USP 1000 mcg/mL - NDC 69680-112-101 mL VialBoxes of 10 - NDC 69680-112-251 mL VialBoxes of 25 - NDC 69680-113-9910 mL Multiple Dose VialBoxes of 10 - NDC ...
-
SPL UNCLASSIFIED SECTIONProduct of France - Manufactured for: Vitruvias Therapeutics - Auburn, Alabama 36830 - Code No.: I112 0923R5 - Revised: 02/2024 - To request additional information or if you have questions concerning this ...
-
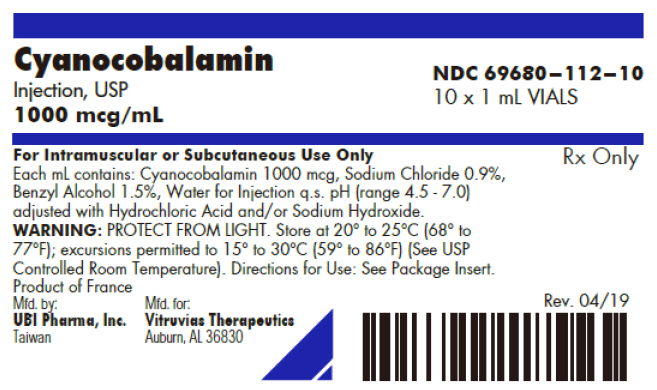
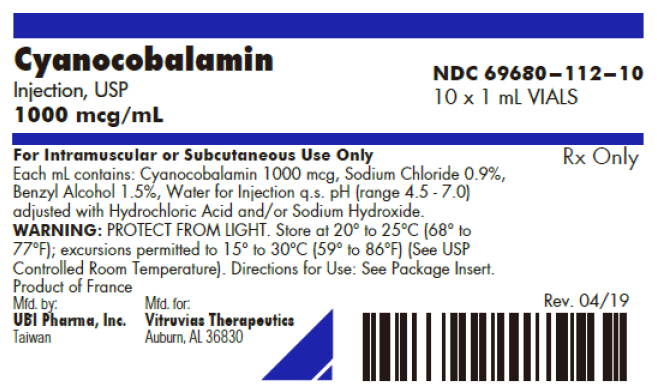
PRINCIPAL DISPLAY PANEL - 1 mL Vial Box LabelCyanocobalamin - Injection, USP - 1000 mcg/mL - NDC 69680–112–10 - 10 x 1 mL VIALS - For Intramuscular or Subcutaneous Use Only - Each mL contains: Cyanocobalamin 1000 mcg, Sodium Chloride 0.9%, Benzyl ...
-
PRINCIPAL DISPLAY PANEL - 10 mL Vial BoxCyanocobalamin - Injection, USP - 10,000 mcg/10 mL (1,000 mcg/mL) NDC 69680–113–99 - 10 x 10 mL - MULTIPLE DOSE VIALS - For Intramuscular or Subcutaneous Use Only - Each mL contains: Cyanocobalamin 1000 mcg ...
-
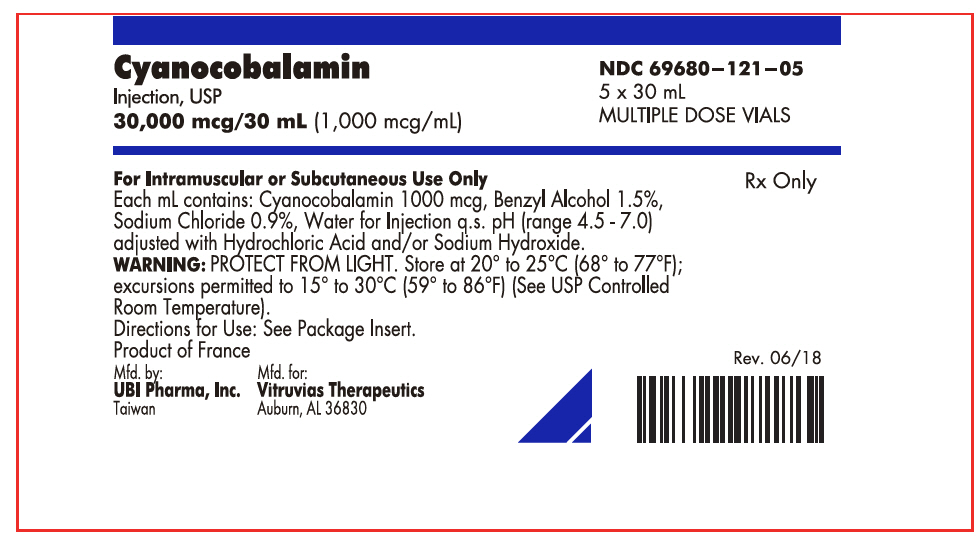
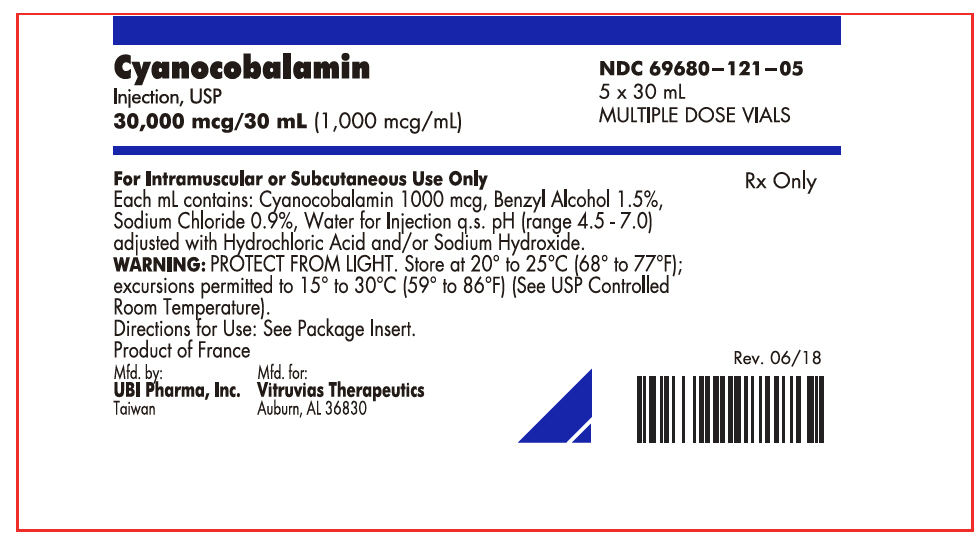
PRINCIPAL DISPLAY PANEL - 30 mL Vial BoxCyanocobalamin - Injection, USP - 30,000 mcg/30 mL (1,000 mcg/mL) NDC 69680–121–05 - 5 x 30 mL - MULTIPLE DOSE VIALS - For Intramuscular or Subcutaneous Use Only - Each mL contains: Cyanocobalamin 1000 mcg ...
-
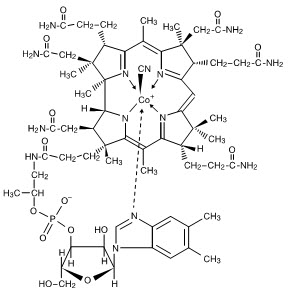
INGREDIENTS AND APPEARANCEProduct Information