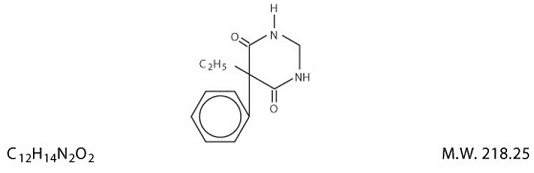
Primidone Tablets, USP, 50 mg and 250 mg
-
Dispense with Medication Guide available at: https://www.oxford-rx.com/med-guides
- Read this Medication Guide before you start taking primidone tablets ...
Primidone Tablets, USP, 50 mg and 250 mg
Dispense with Medication Guide available at: https://www.oxford-rx.com/med-guides
Read this Medication Guide before you start taking primidone tablets and each time you get a refill. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or treatment.
What is the most important information I should know about primidone tablets?.
Like other antiepileptic drugs, primidone tablets may cause suicidal thoughts or actions in a very small number of people, about 1 in 500.
Call a healthcare provider right away if you have any of these symptoms, especially if they are new, worse, or worry you:
- thoughts about suicide or dying
- attempts to commit suicide
- new or worse depression
- new or worse anxiety
- feeling agitated or restless
- panic attacks
- trouble sleeping (insomnia)
- new or worse irritability
- acting aggressive, being angry, or violent
- acting on dangerous impulses
- an extreme increase in activity and talking (mania)
- other unusual changes in behavior or mood
Do not stop primidone tablets without first talking to a healthcare provider.
- Stopping primidone tablets suddenly can cause serious problems. Stopping a seizure medicine suddenly in a patient who has epilepsy can cause seizures that will not stop (status epilepticus).
Suicidal thoughts or actions can be caused by things other than medicines. If you have suicidal thoughts or actions, your healthcare provider may check for other causes.
How can I watch for early symptoms of suicidal thoughts and actions?
- Pay attention to any changes, especially sudden changes, in mood, behaviors, thoughts, or feelings.
- Keep all follow-up visits with your healthcare provider as scheduled.
- Call your healthcare provider between visits as needed, especially if you are worried about symptoms.
What are primidone tablets?
Primidone tablets are a prescription medicine used alone or with other medicines to treat people with:
- generalized tonic-clonic (grand mal) seizures
- complex partial (psychomotor) seizures
- partial (focal) epileptic seizures.
Who should not take primidone tablets?
Do not take primidone tablets if you:
- have a genetic disorder called porphyria
- are allergic to phenobarbital
What should I tell my healthcare provider before taking primidone tablets?
Before you take primidone tablets, tell your healthcare provider if you:
- have or have had depression, mood problems or suicidal thoughts or behavior
- have any other medical conditions
- are pregnant or planning to become pregnant. Primidone tablets may harm your unborn baby. Tell your healthcare provider right away if you become pregnant while taking primidone tablets. You and your healthcare provider will decide if you should take primidone tablets while you are pregnant.
- If you become pregnant while taking primidone tablets, talk to your healthcare provider about registering with the North American Antiepileptic Drug (NAAED) Pregnancy Registry. You can enroll in this registry by calling 1-888-233-2334. The purpose of this registry is to collect information about the safety of antiepileptic drugs during pregnancy.
- are breastfeeding or plan to breastfeed. Primidone can pass into breast milk. Talk to your healthcare provider about the best way to feed your baby if you take primidone tablets.
Tell your healthcare provider about all the medicines you take, including prescription and non-prescription medicines, vitamins, and herbal supplements. Taking primidone tablets with certain other medicines can cause side effects or affect how well they work. Do not start or stop other medicines without talking to your healthcare provider.
Know the medicines you take. Keep a list of them and show it to your healthcare provider and pharmacist each time you get a new medicine.
How should I take primidone tablets?
Take primidone tablets exactly as prescribed. Your healthcare provider will tell you how much primidone tablets to take and when to take it.
- Your healthcare provider may change your dose. Do not change your dose without talking to your healthcare provider.
- Do not stop taking primidone tablets without first talking to your healthcare provider. Stopping primidone tablets suddenly can cause serious problems.
- If you take too much primidone tablets, call your healthcare provider or local Poison Control Center right away.
What should I avoid while taking primidone tablets?
- Primidone tablets can make you sleepy or dizzy. Do not drink alcohol or take other drugs that make you sleepy or dizzy while taking primidone tablets without first discussing this with your healthcare provider. Taking primidone tablets with alcohol or drugs that cause sleepiness or dizziness may make your sleepiness or dizziness worse.
- Do not drive, operate heavy machinery, or do other dangerous activities until you know how primidone tablets affect you. Primidone tablets can slow your thinking and motor skills.
What are the possible side effects of primidone tablets?
See “What is the most important information I should know about primidone tablets?”.
Primidone tablets may cause other serious side effects including:
- Sleepiness that can be severe, especially when you first start taking primidone tablets.
- Primidone tablets may rarely cause blood problems. Symptoms may include:
- fever, swollen glands, or sore throat that come and go or do not go away
- frequent infections or an infection that does not go away
- tiredness
- shortness of breath
- Primidone tablets may rarely cause allergic reactions. Symptoms may include:
- skin rash
- hives
- sores in your mouth
- blistering or peeling skin
The most common side effects of primidone tablets include:
- problems with walking and moving
- feelings of dizziness, spinning, or swaying (vertigo)
These are not all the possible side effects of primidone tablets. For more information, ask your healthcare provider or pharmacist.
Tell your healthcare provider if you have any side effect that bothers you or that does not go away.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1- 800-FDA-1088.
How should I store primidone tablets?
Store primidone tablets at room temperature between 68°F to 77°F (20°C to 25°C) in a tight, light- resistant container.
Keep primidone tablets and all medicines out of the reach of children.
General Information about primidone tablets
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use primidone tablets for a condition for which it was not prescribed. Do not give primidone tablets to other people, even if they have the same symptoms that you have. It may harm them.
This Medication Guide summarizes the most important information about primidone tablets. If you would like more information, talk with your healthcare provider. You can ask your pharmacist or healthcare provider for information about primidone tablets that is written for health professionals.
For more information, call 1-844-508-1455, 8:00 AM to 4.30 PM ET, Monday – Friday.
What are the ingredients in Primidone?
Active Ingredient: primidone
Inactive ingredients: hypromellose, lactose monohydrate, magnesium stearate, microcrystalline cellulose, sodium lauryl sulfate, sodium starch glycolate and talc.
Primidone 250 mg tablets also contain D&C yellow #10 aluminum lake and FD&C yellow #5 aluminum lake.
Primidone Tablets 250 mg contain FD&C Yellow No. 5 (tartrazine) which may cause allergic-type reactions (including bronchial asthma) in certain susceptible persons. Although the overall incidence of FD&C Yellow No. 5 (tartrazine) sensitivity in the general population is low, it is frequently seen in patients who also have aspirin hypersensitivity.
This Medication Guide has been approved by the U.S. Food and Drug Administration.
Manufactured by:
OXFORD PHARMACEUTICALS, LLC
Birmingham, AL 35211
8200023 Rev 01
01/22
Close