Label: GLIMEPIRIDE tablet
- NDC Code(s): 69543-123-10, 69543-123-50, 69543-124-10, 69543-124-50, view more
- Packager: Virtus Pharmaceuticals
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated March 22, 2019
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use GLIMEPIRIDE TABLETS safely and effectively. See full prescribing information for GLIMEPIRIDE TABLETS. GLIMEPIRIDE tablets, for oral ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE Glimepiride tablets are indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus [see Clinical Studies (14.1)]. Limitations of ...
-
2 DOSAGE AND ADMINISTRATION 2.1 Recommended Dosing - Glimepiride tablets should be administered with breakfast or the first main meal of the day. The recommended starting dose of glimepiride tablets is 1 mg or 2 mg once ...
-
3 DOSAGE FORMS AND STRENGTHS Glimepiride tablets USP are formulated as tablets of: • 1 mg (Light pink capsule shaped flat-faced, bevelled edged tablets with breakline on both sides and debossed with ‘I’ and ‘1’ on either ...
-
4 CONTRAINDICATIONS Glimepiride tablets are contraindicated in patients with a history of a hypersensitivity reaction to: • Glimepiride or any of the product's ingredients [see Warnings and Precautions ...
-
5 WARNINGS AND PRECAUTIONS 5.1 Hypoglycemia - All sulfonylureas, including glimepiride, can cause severe hypoglycemia [see Adverse Reactions (6.1)]. The patient's ability to concentrate and react may be impaired as a ...
-
6 ADVERSE REACTIONS The following serious adverse reactions are discussed in more detail below and elsewhere in the labeling: • Hypoglycemia [see Warnings and Precautions (5.1)] • Hemolytic anemia [see Warnings and ...
-
7 DRUG INTERACTIONS 7.1 Drugs Affecting Glucose Metabolism - A number of medications affect glucose metabolism and may require glimepiride dose adjustment and particularly close monitoring for hypoglycemia or ...
-
8 USE IN SPECIFIC POPULATIONS 8.1 Pregnancy - Risk Summary - Available data from a small number of published studies and postmarketing experience with glimepiride tablets use in pregnancy over decades have not identified any ...
-
10 OVERDOSAGE An overdosage of glimepiride, as with other sulfonylureas, can produce severe hypoglycemia. Mild episodes of hypoglycemia can be treated with oral glucose. Severe hypoglycemic reactions constitute ...
-
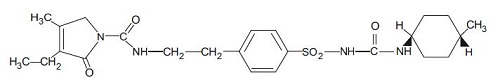
11 DESCRIPTION Glimepiride tablets USP is an oral sulfonylurea that contains the active ingredient glimepiride. Chemically, glimepiride is identified as ...
-
12 CLINICAL PHARMACOLOGY 12.1 Mechanism of Action - Glimepiride primarily lowers blood glucose by stimulating the release of insulin from pancreatic beta cells. Sulfonylureas bind to the sulfonylurea receptor in the ...
-
13 NONCLINICAL TOXICOLOGY 13.1 Carcinogenesis, Mutagenesis, and Impairment of Fertility - Studies in rats at doses of up to 5000 parts per million (ppm) in complete feed (approximately 340 times the maximum recommended ...
-
14 CLINICAL STUDIES 14.1 Monotherapy - A total of 304 patients with type 2 diabetes already treated with sulfonylurea therapy participated in a 14-week, multicenter, randomized, double-blind, placebo-controlled ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING Glimepiride tablets USP are available in the following strengths and package sizes: • 1 mg (Light pink capsule shaped flat-faced, bevelled edged tablets with breakline on both sides and debossed ...
-
17 PATIENT COUNSELING INFORMATION Hypoglycemia - Explain the symptoms and treatment of hypoglycemia as well as conditions that predispose to hypoglycemia. Inform patients that their ability to concentrate and react may be impaired ...
-
PRINCIPAL DISPLAY PANEL - 1 mg Tablet Bottle Label NDC 69543-123-50 - Glimepiride Tablets - USP - 1 mg - Rx ONLY - ONCE A DAY - 500 Tablets
-
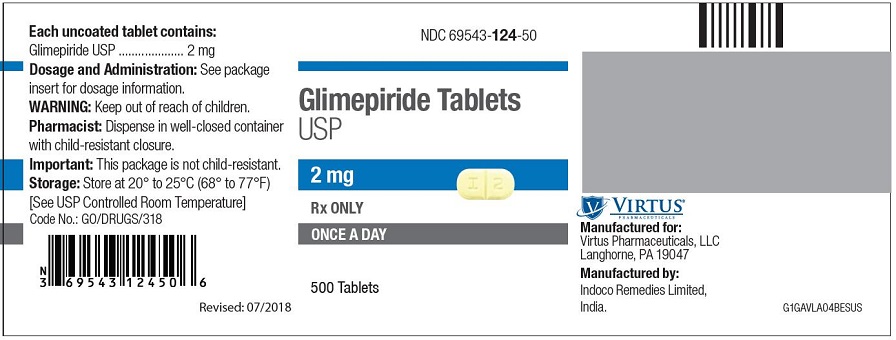
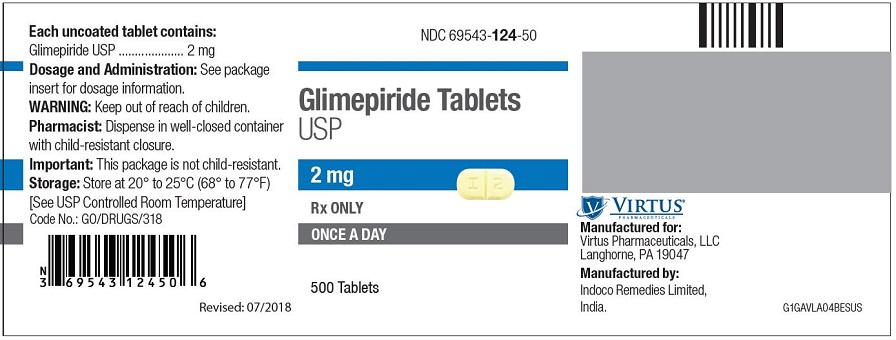
PRINCIPAL DISPLAY PANEL - 2 mg Tablet Bottle Label NDC 69543-124-50 - Glimepiride Tablets - USP - 2 mg - Rx ONLY - ONCE A DAY - 500 Tablets
-
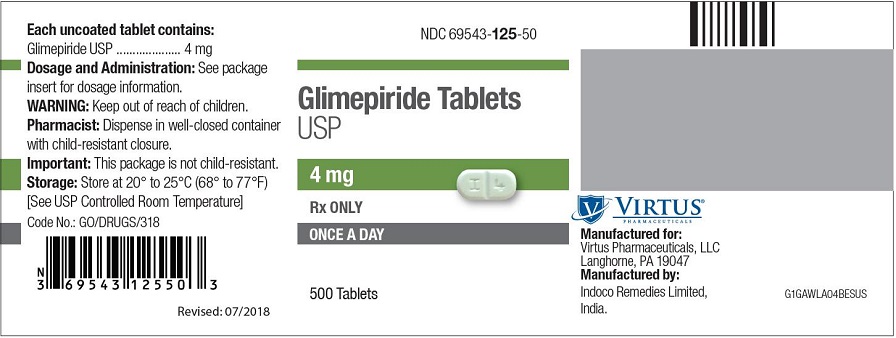
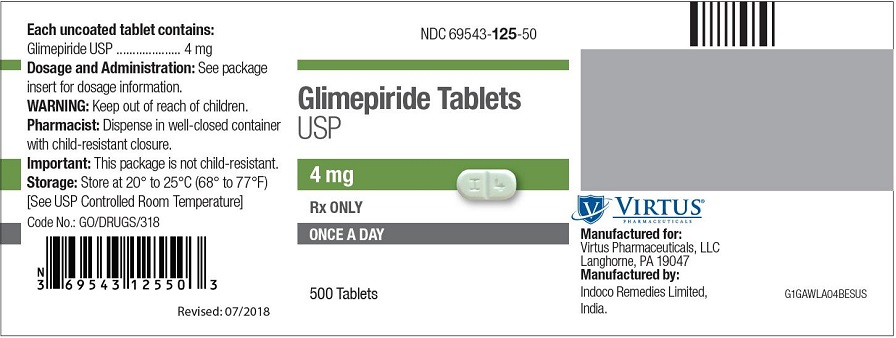
PRINCIPAL DISPLAY PANEL - 4 mg Tablet Bottle Label NDC 69543-125-50 - Glimepiride Tablets - USP - 4 mg - Rx ONLY - ONCE A DAY - 500 Tablets
-
INGREDIENTS AND APPEARANCEProduct Information