Label: OZOBAX DS- baclofen solution
- NDC Code(s): 69528-302-08, 69528-302-16
- Packager: Metacel Pharmaceuticals, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated October 17, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use OZOBAX® DS safely and effectively. See full prescribing information for OZOBAX DS. OZOBAX DS (baclofen) oral solution ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEOZOBAX DS is indicated for the treatment of spasticity resulting from multiple sclerosis, particularly for the relief of flexor spasms and concomitant pain, clonus, and muscular rigidity. OZOBAX ...
-
2 DOSAGE AND ADMINISTRATION2.1 Important Dosage Information - Baclofen oral solution is available in multiple concentrations; ensure accuracy when prescribing, dispensing and administering baclofen oral solution to avoid ...
-
3 DOSAGE FORMS AND STRENGTHSOral Solution: 10 mg/5 mL baclofen as a clear, colorless solution
-
4 CONTRAINDICATIONSOZOBAX DS is contraindicated in patients with hypersensitivity to baclofen.
-
5 WARNINGS AND PRECAUTIONS5.1 Adverse Reactions from Abrupt Withdrawal of OZOBAX DS - Abrupt discontinuation of baclofen, regardless of the cause, has resulted in adverse reactions that include hallucinations, seizures ...
-
6 ADVERSE REACTIONSThe following clinically significant adverse reactions are described elsewhere in the labeling: Adverse Reactions from Abrupt Withdrawal of OZOBAX DS [see Warnings and Precautions ( 5.1) ...
-
7 DRUG INTERACTIONS7.1 CNS Depressants and Alcohol - OZOBAX DS can cause CNS depression, including drowsiness and sedation, which may be additive when used concomitantly with other CNS depressants or alcohol [see ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - There are no adequate data on the developmental risk associated with the use of OZOBAX DS in pregnant women. Oral administration of baclofen to pregnant rats ...
-
10 OVERDOSAGE10.1 Symptoms of Baclofen Overdose - Patients may present in coma or with progressive drowsiness, lightheadedness, dizziness, somnolence, accommodation disorders, respiratory depression ...
-
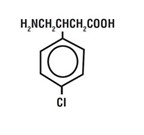
11 DESCRIPTIONOZOBAX DS (baclofen) oral solution is a gamma-aminobutyric acid (GABA-ergic) agonist available as 10 mg/5 mL solution for oral administration. Its chemical name is ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - The precise mechanism of action of baclofen is not fully understood. Baclofen inhibits both monosynaptic and polysynaptic reflexes at the spinal level, possibly by ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - No increase in tumors was seen in rats receiving baclofen orally for two years at approximately 30 to 60 times on a ...
-
14 CLINICAL STUDIESThe efficacy of OZOBAX DS is based upon a bioavailability study in healthy adults comparing baclofen oral tablets to baclofen oral solution [see Clinical Pharmacology ( 12.3)].
-
16 HOW SUPPLIED/STORAGE AND HANDLING16.1 How Supplied - OZOBAX DS (baclofen) Oral Solution contains 10 mg/5 mL baclofen. It is a clear, colorless solution and is supplied in bottles of 237 mL (NDC 69528-302-08) and 473 mL (NDC ...
-
17 PATIENT COUNSELING INFORMATIONAdministration Instructions - Instruct patients or caregivers to use an oral dosing syringe to correctly measure the prescribed amount of medication. Inform patients that oral dosing syringes may ...
-
PACKAGE LABEL. PRINCIPAL DISPLAY PANELNDC 69528-302-08 - OZOBAX ® DS - (baclofen) Oral Solution - 10 mg / 5 mL (2 mg/mL) Baclofen oral solution is available in different concentrations - Clear liqud solution - RX only - 8 fl. oz. (237 ...
-
INGREDIENTS AND APPEARANCEProduct Information