Label: TERAZOSIN capsule
- NDC Code(s): 69452-330-20, 69452-330-32, 69452-331-20, 69452-331-32, view more
- Packager: Bionpharma Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated December 20, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
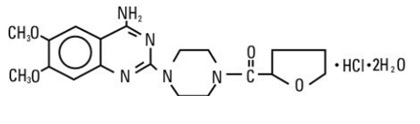
DESCRIPTIONTerazosin hydrochloride, USP an alpha-1-selective adrenoceptor blocking agent, is a quinazoline derivative represented by the following chemical name and structural formula ...
-
CLINICAL PHARMACOLOGYPharmacodynamics - A. Benign Prostatic Hyperplasia (BPH) The symptoms associated with BPH are related to bladder outlet obstruction, which is comprised of two underlying components: a ...
-
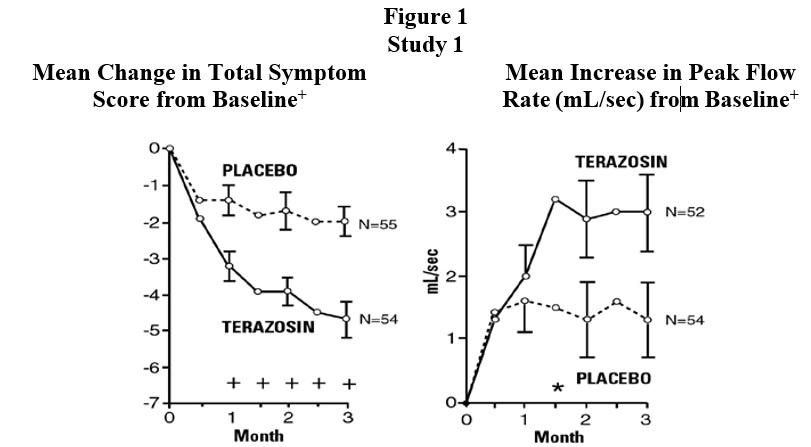
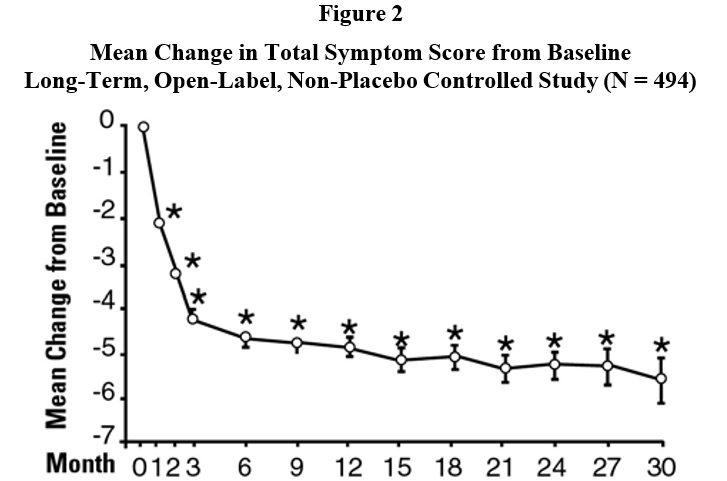
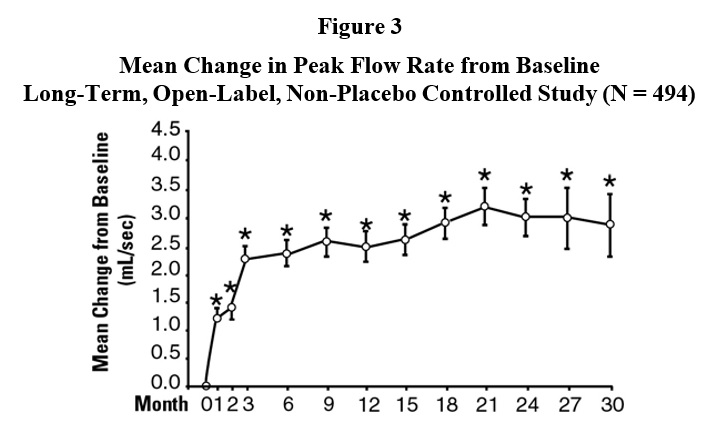
INDICATIONS AND USAGETerazosin capsules are indicated for the treatment of symptomatic benign prostatic hyperplasia (BPH). There is a rapid response, with approximately 70% of patients experiencing an increase in ...
-
CONTRAINDICATIONSTerazosin capsules are contraindicated in patients known to be hypersensitive to terazosin hydrochloride.
-
WARNINGSSyncope and "First-dose" Effect - Terazosin capsules, like other alpha-adrenergic blocking agents, can cause marked lowering of blood pressure, especially postural hypotension, and syncope in ...
-
PRECAUTIONSGeneral - Prostatic Cancer - Carcinoma of the prostate and BPH cause many of the same symptoms. These two diseases frequently co-exist. Therefore, patients thought to have BPH should be examined ...
-
Drug InteractionsIn controlled trials, terazosin has been added to diuretics, and several beta-adrenergic blockers; no unexpected interactions were observed. Terazosin has also been used in patients on a variety ...
-
PregnancyTeratogenic effects: Terazosin was not teratogenic in either rats or rabbits when administered at oral doses up to 280 times and 60 times, respectively, the maximum recommended human dose. Fetal ...
-
ADVERSE REACTIONSBenign Prostatic Hyperplasia - The incidence of treatment-emergent adverse events has been ascertained from clinical trials conducted worldwide. All adverse events reported during these trials ...
-
OVERDOSAGEShould overdosage of terazosin lead to hypotension, support of the cardiovascular system is of first importance. Restoration of blood pressure and normalization of heart rate may be accomplished ...
-
DOSAGE AND ADMINISTRATIONIf terazosin capsules administration is discontinued for several days, therapy should be reinstituted using the initial dosing regimen. Benign Prostatic Hyperplasia - Initial Dose - 1 mg at ...
-
HOW SUPPLIEDTerazosin capsules, USP are available in four dosage strengths - 1 mg gray capsules available as hard gelatin capsule with gray opaque cap and gray opaque body filled with white to off white powder ...
-
PATIENT INFORMATION ABOUTTerazosin (ter-A-zo-sin) Capsules, USP - When used to treat HYPERTENSION or BENIGN PROSTATIC HYPERPLASIA (BPH) Please read this leaflet before you start taking terazosin capsules. Also ...
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANELNDC 69452-330-20 - Terazosin Capsules, USP - 1 mg - Rx only - 100 Capsules - NDC 69452-331-20 - Terazosin Capsules, USP - 2 mg - Rx only - 100 Capsules - NDC 69452-332-20 - Terazosin ...
-
1 mg-1000's countNDC 69452-330-32 - Terazosin - Capsules, USP - 1 mg - Rx only - 1000 Capsules
-
2 mg- 1000's countNDC 69452-331-32 - Terazosin - Capsules, USP - 2 mg - Rx only - 1000 Capsules
-
5 mg- 1000's countNDC 69452-332-32 - Terazosin - Capsules, USP - 5 mg - Rx only 1000 Capsules
-
10 mg- 1000's countNDC 69452-333-32 - Terazosin - Capsules, USP - 10 mg - Rx only 1000 Capsules
-
INGREDIENTS AND APPEARANCEProduct Information