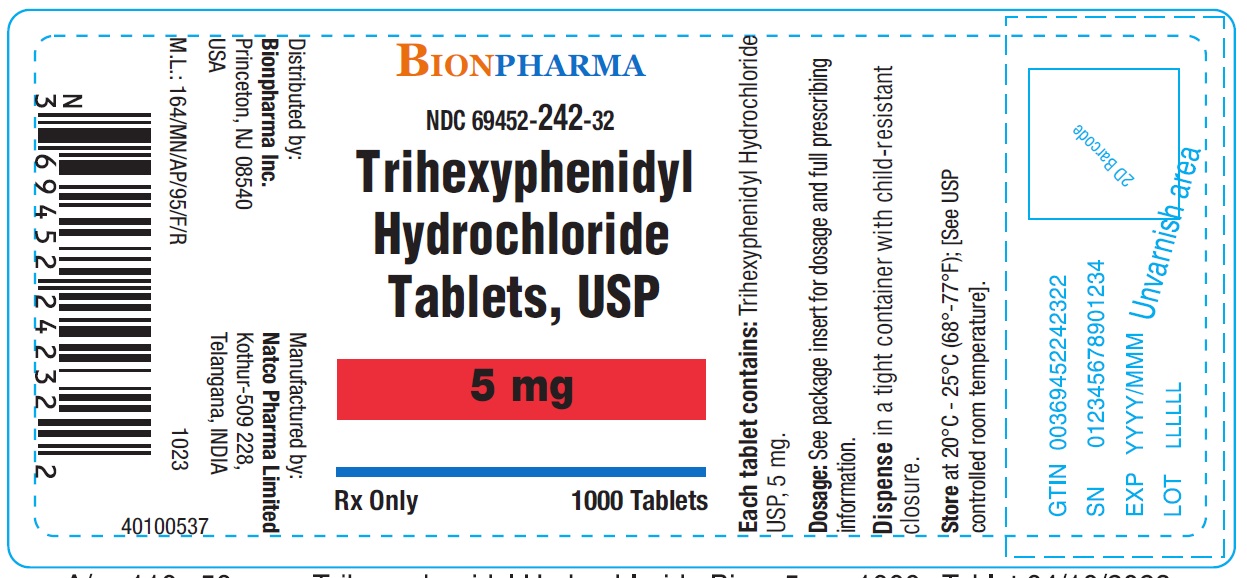
Label: TRIHEXYPHENIDYL HYDROCHLORIDE tablet
- NDC Code(s): 69452-241-20, 69452-241-32, 69452-242-20, 69452-242-32
- Packager: Bionpharma Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated January 12, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONRx Only
-
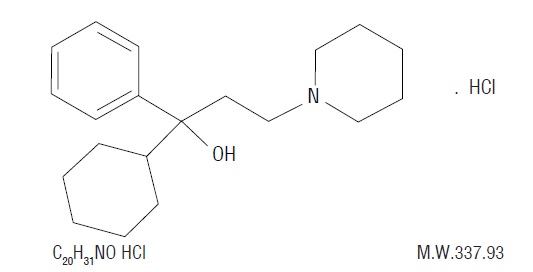
DESCRIPTIONTrihexyphenidyl HCl is a synthetic antispasmodic drug. It is designated chemically as α-Cyclohexylα-phenyl-1-piperidinepropanol hydrochloride and its structural formula is as ...
-
CLINICAL PHARMACOLOGYTrihexyphenidyl HCl exerts a direct inhibitory effect upon the parasympathetic nervous system. It also has a relaxing effect on smooth musculature; exerted both directly upon the muscle tissue ...
-
INDICATIONS AND USAGETrihexyphenidyl HCl is indicated as an adjunct in the treatment of all forms of parkinsonism (postencephalitic, arteriosclerotic, and idiopathic). It is often useful as adjuvant therapy when ...
-
CONTRAINDICATIONSTrihexyphenidyl HCl is contraindicated in patients with hypersensitivity to trihexyphenidyl HCl or to any of the tablet ingredients. Trihexyphenidyl HCl is also contraindicated in patients with ...
-
WARNINGSPatients to be treated with trihexyphenidyl HCl should have a gonioscope evaluation prior to initiation of therapy and close monitoring of intraocular pressures. The use of anticholinergic drugs ...
-
PRECAUTIONSGeneral - Patients with cardiac, liver, or kidney disorders, or with hypertension, should be closely monitored. Since trihexyphenidyl HCl has atropine-like properties, patients on long-term ...
-
ADVERSE REACTIONSMinor side effects, such as dryness of the mouth, blurred vision, dizziness, mild nausea or nervousness, will be experienced by 30 to 50 percent of all patients. These sensations, however, are ...
-
DRUG ABUSE AND DEPENDENCEAlthough trihexyphenidyl HCl is not classified as a controlled substance, the possibility of abuse should be borne in mind due to its stimulant and euphoriant properties.
-
OVERDOSAGEThe mean oral LD50 of trihexyphenidyl HCl has been reported to be 365 mg/kg (range, 325 to 410 mg/kg) in mice and 1660 mg/kg (1420 to 1940 mg/kg) in rats. At a dose of 40 mg/kg, dogs have ...
-
DOSAGE AND ADMINISTRATIONDosage should be individualized. The initial dose should be low and then increased gradually, especially in patients over 60 years of age. Whether trihexyphenidyl HCl may best be given before or ...
-
HOW SUPPLIEDTrihexyphenidyl Hydrochloride Tablets, USP 2 mg are white colored, round debossed with N, T on either side of the score line and ‘2’ on the other side. Trihexyphenidyl Hydrochloride Tablets, USP 2 ...
-
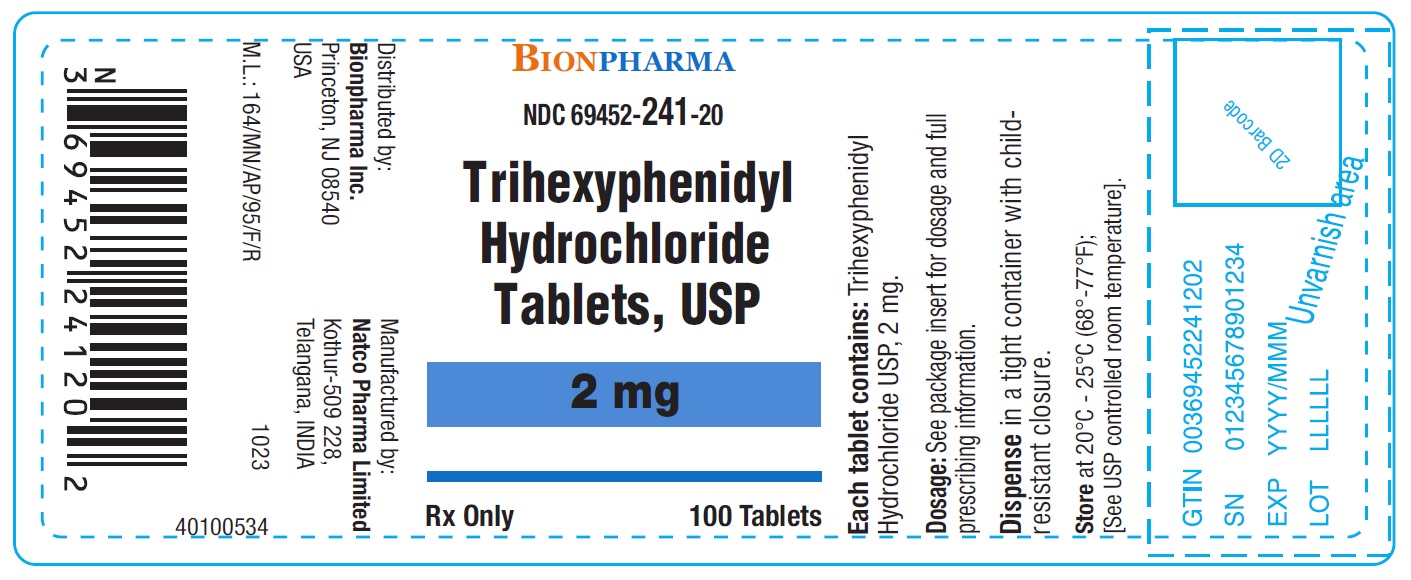
Trihexyphenidyl Hydrochloride Tablets USP 2 mg-Bottle of 100NDC 69452-241-20 - Each tablet contains Trihexyphenidyl hydrochloride USP 2 mg - Dispense in tight container with child-resistant closure.
-
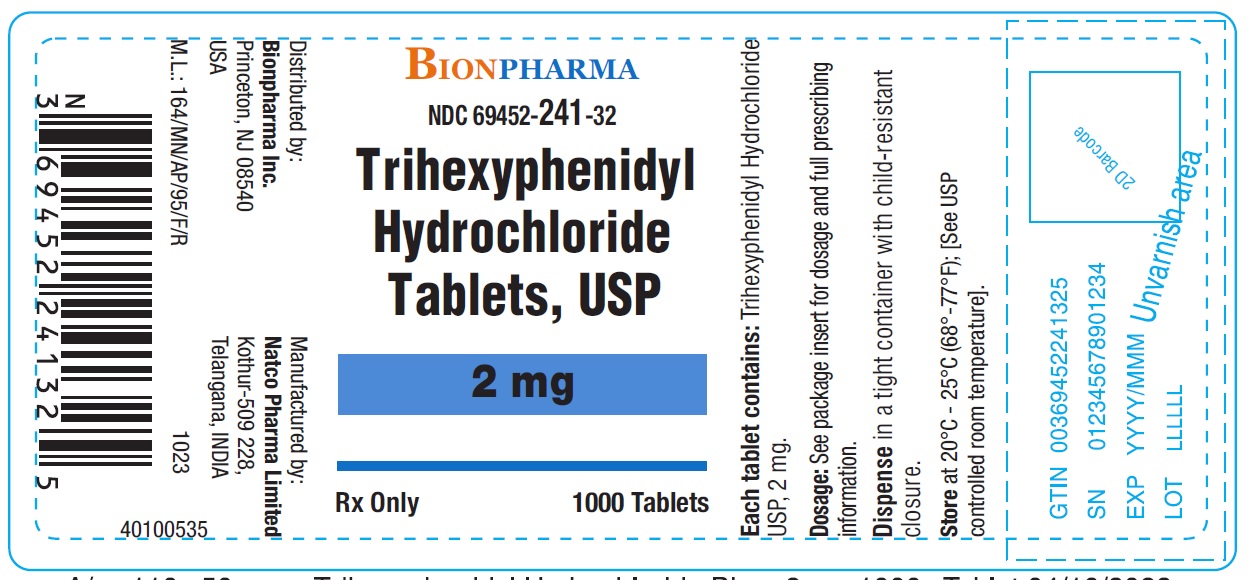
Trihexyphenidyl Hydrochloride Tablets USP 2 mg-Bottle of 1000NDC 69452-241-32 - Each tablet contains Trihexyphenidyl hydrochloride USP 2 mg - Dispense in tight container with child-resistant closure.
-
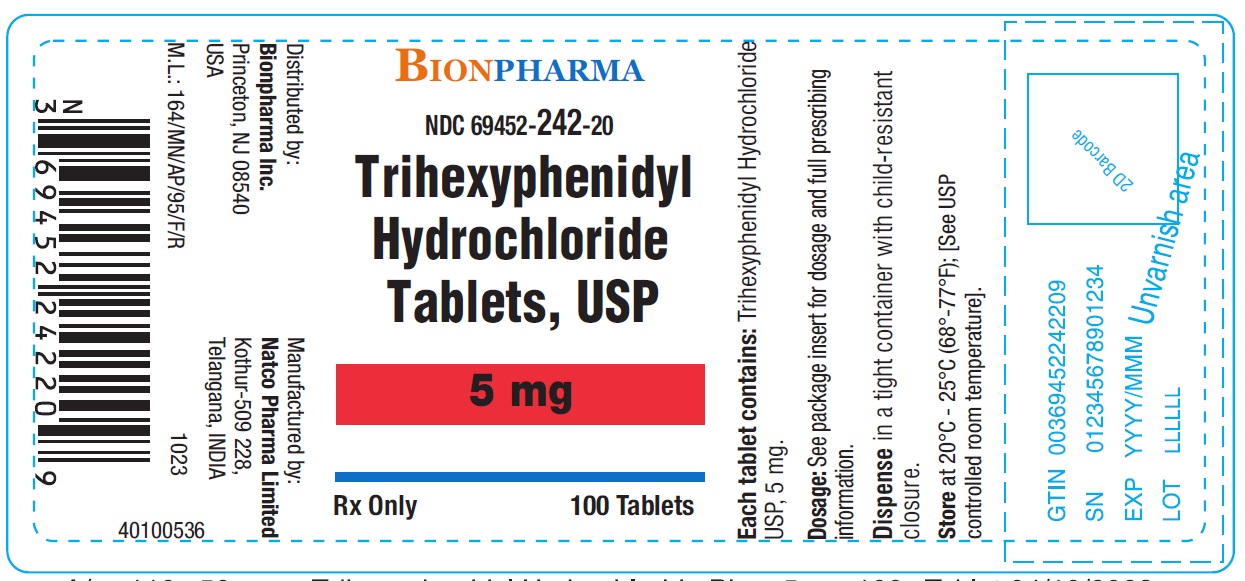
Trihexyphenidyl Hydrochloride Tablets USP 5 mg-Bottle of 100NDC 69452-242-20 - Each tablet contains Trihexyphenidyl hydrochloride USP 5 mg - Dispense in tight container with child-resistant closure.
-
Trihexyphenidyl Hydrochloride Tablets USP 5 mg-Bottle of 1000NDC 69452-242-32 - Each tablet contains Trihexyphenidyl hydrochloride USP 5 mg - Dispense in tight container with child-resistant closure.
-
INGREDIENTS AND APPEARANCEProduct Information