Label: RUFINAMIDE suspension
- NDC Code(s): 69452-223-84
- Packager: Bionpharma Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated March 5, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use RUFINAMIDE ORAL SUSPENSIONsafely and effectively. See full prescribing information for RUFINAMIDE ORAL SUSPENSION. RUFINAMIDE ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGERufinamide oral suspension is indicated for adjunctive treatment of seizures associated with Lennox-Gastaut Syndrome in pediatric patients 1 year of age and older and in adults.
-
2 DOSAGE AND ADMINISTRATION2.1 Dosage Information - Pediatric patients(1 year to less than 17 years) The recommended starting daily dose of rufinamide oral suspension in pediatric patients with Lennox-Gastaut Syndrome is ...
-
3 DOSAGE FORMS AND STRENGTHSOral Suspension: 40 mg/mL. White to off-white, orange flavored liquid.
-
4 CONTRAINDICATIONSRufinamide is contraindicated in patients with Familial Short QT syndrome - [see Warnings and Precautions (5.3)].
-
5 WARNINGS AND PRECAUTIONS5.1 Suicidal Behavior and Ideation - Antiepileptic drugs (AEDs), including rufinamide, increase the risk of suicidal thoughts or behavior in patients taking these drugs for any indication ...
-
6 ADVERSE REACTIONSThe following serious adverse reactions are described below and elsewhere in the labeling: Suicidal Behavior and Ideation - [see Warnings and Precautions (5.1)] Central Nervous System ...
-
7 DRUG INTERACTIONS7.1 Effects of Rufinamide on other AEDs - Population pharmacokinetic analysis of average concentration at steady state of carbamazepine, lamotrigine, phenobarbital, phenytoin, topiramate, and ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Pregnancy Exposure Registry - There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to AEDs, such as rufinamide, during pregnancy. Encourage ...
-
10 OVERDOSAGEBecause strategies for the management of overdose are continually evolving, it is advisable to contact a Certified Poison Control Center to determine the latest recommendations for the management ...
-
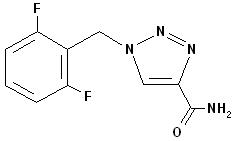
11 DESCRIPTIONRufinamide, USP is a triazole derivative structurally unrelated to currently marketed antiepileptic drugs (AEDs). Rufinamide, USP has the chemical name ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - The precise mechanism(s) by which rufinamide exerts its antiepileptic effect is unknown. The results of - in vitrostudies suggest that the principal mechanism of ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - Rufinamide was given in the diet to mice at 40, 120, and 400 mg/kg per day and to rats at 20, 60, and 200 mg/kg per ...
-
14 CLINICAL STUDIESAdult and Pediatric Patients ages 4 years and older - The effectiveness of rufinamide as adjunctive treatment for the seizures associated with Lennox-Gastaut Syndrome (LGS) in adult and pediatric ...
-
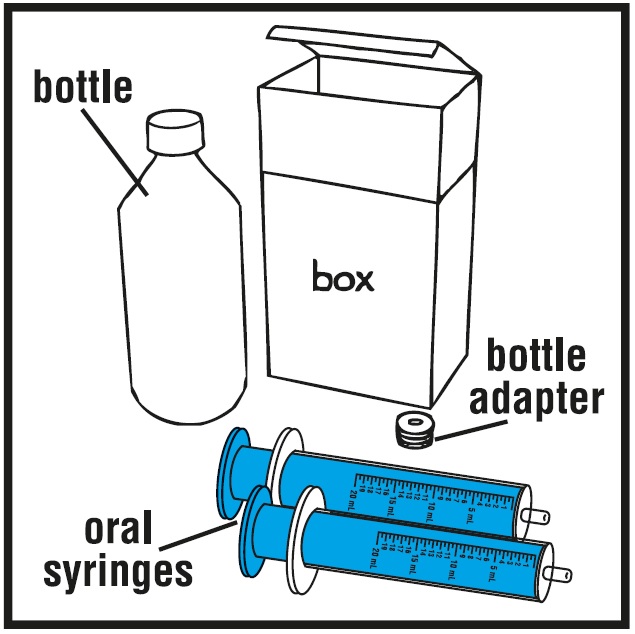
16 HOW SUPPLIED/STORAGE AND HANDLING16.1 How Supplied - Rufinamide oral suspension is a white to off-white, orange flavored liquid supplied in an amber polyethylene terephthalate (PET) bottle with child-resistant closure. The oral ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Medication Guide and Instructions for Use). Administration Information - Advise patients to take rufinamide oral suspension with food ...
-
MEDICATION GUIDEMedication Guide - Rufinamide (roo fin’ a mide) Oral Suspension - Read this Medication Guide before you start taking rufinamide oral suspension and each time you get a refill. There may be new ...
-
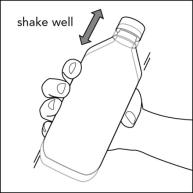
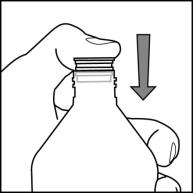
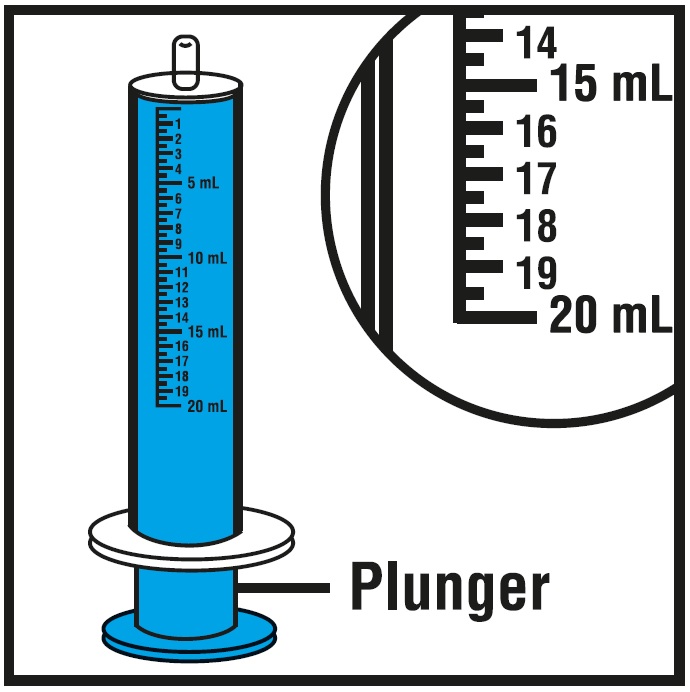
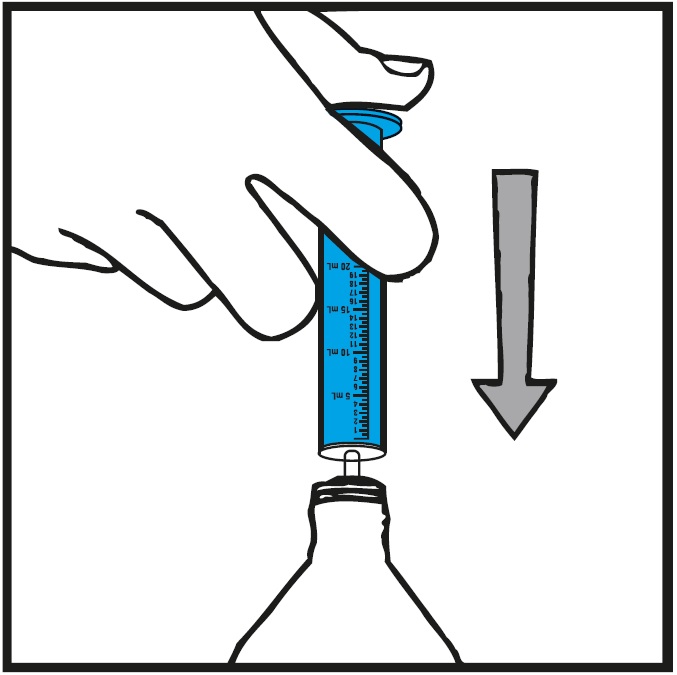
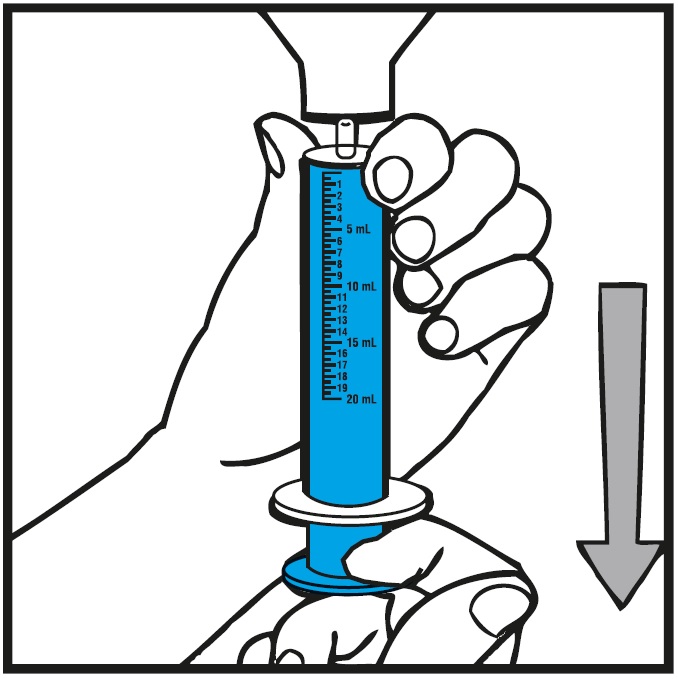
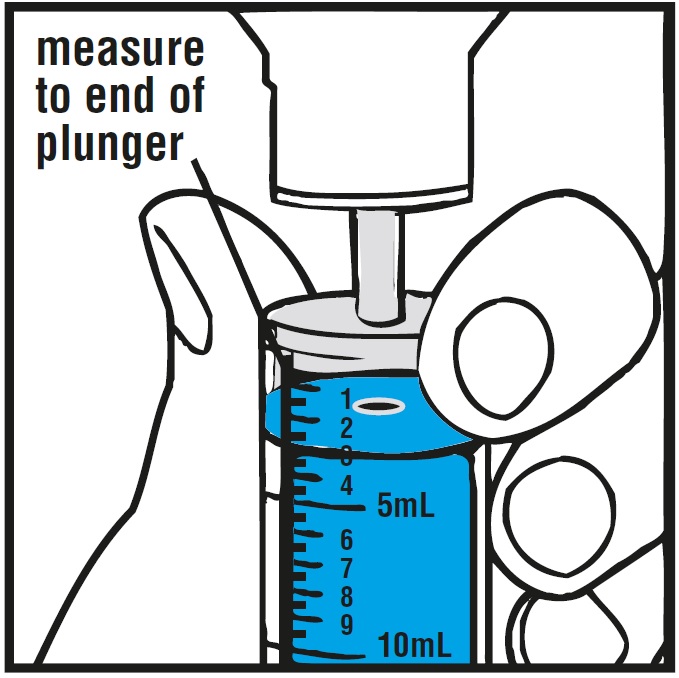
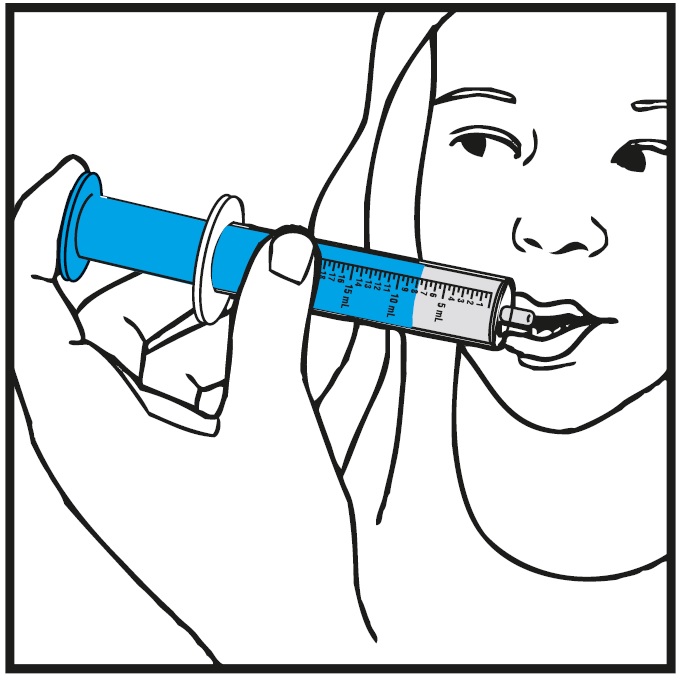
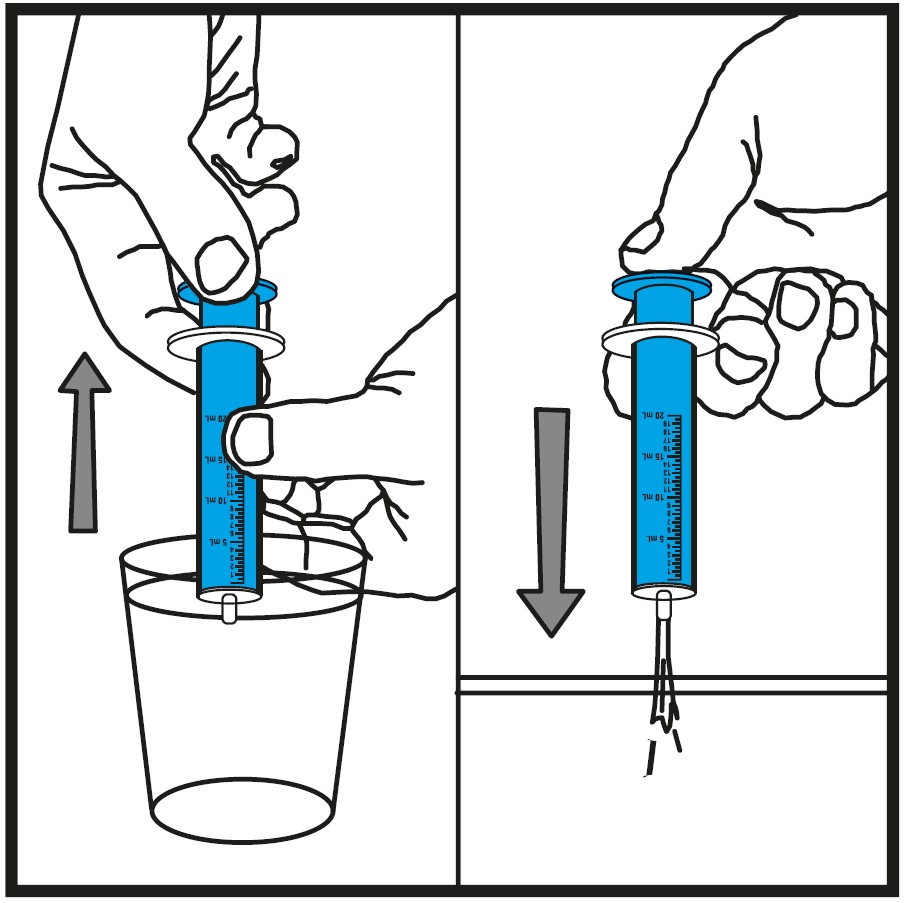
INSTRUCTIONS FOR USEInstructions for Use - Rufinamide (roo fin’ a mide) Oral Suspension - Read the Instructions for Use before using rufinamide oral suspension and each time you get a refill. There may be new ...
-
PRINCIPAL DISPLAY PANELPRINCIPAL DISPLAY PANEL- 40 mg/mL ORAL SUSPENSION - NDC 69452-223-84 - Rufinamide Oral Suspension - 40 mg/mL - For Oral Administration Only - This product is an oral suspension liquid and is ...
-
INGREDIENTS AND APPEARANCEProduct Information