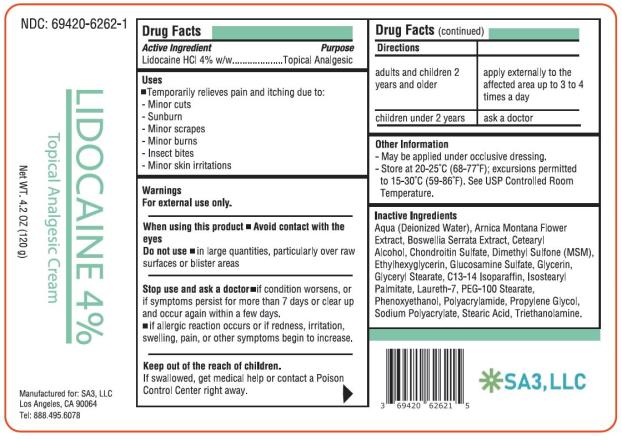
Label: LIDOCAINE 4 PERCENT- lidocaine cream
- NDC Code(s): 69420-6262-1
- Packager: SA3, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 9, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONLIDOCAINE - Lidocaine HCl 4% Cream - SA3, LLC - Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and ...
-
Active ingredient
Lidocaine HCl 4% w/w
-
Purpose
Topical Analgesic
-
Uses
Temporarily relieves pain and itching due to: minor cuts - sunburn - minor scrapes - minor burns - insect bites - minor skin irritations
-
Warnings
For external use only. When using this product - Avoid contact with the eyes - Do not use in large quantities, particularly over raw surfaces or blistered areas - Stop use and ask a ...
-
Directions
adults and children 2 years and olderapply externally to the affected area up to 3 to 4 times a day - children under 2 years ask a doctor
-
Other information
May be applied under occlusive dressing. Store at 20-25°C (68-77°F); excursions permitted to 15-30°C (59-86°F). See USP Controlled Room Temperature.
-
Inactive ingredients
Aqua (Deionized Water), Arnica Montana Flower Extract, Boswellia Serrata Extract, Cetearyl Alcohol, Chondroitin Sulfate, Dimethyl Sulfone (MSM), Ethylhexylglycerin, Glucosamine Sulfate, Glycerin ...
-
PRINCIPAL DISPLAY PANEL
Lidocaine HCl 4% cream - NDC 69420-6262-1 - Topical Analgesic Cream - 4.2 OZ (120 g) SA3, LLC
-
INGREDIENTS AND APPEARANCEProduct Information