Label: CAPSAICIN cream
- NDC Code(s): 69420-6025-1, 69420-6025-5
- Packager: SA3, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 9, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONCAPSAICIN – Capsaicin 0.025% Cream - SA3, LLC - Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and ...
-
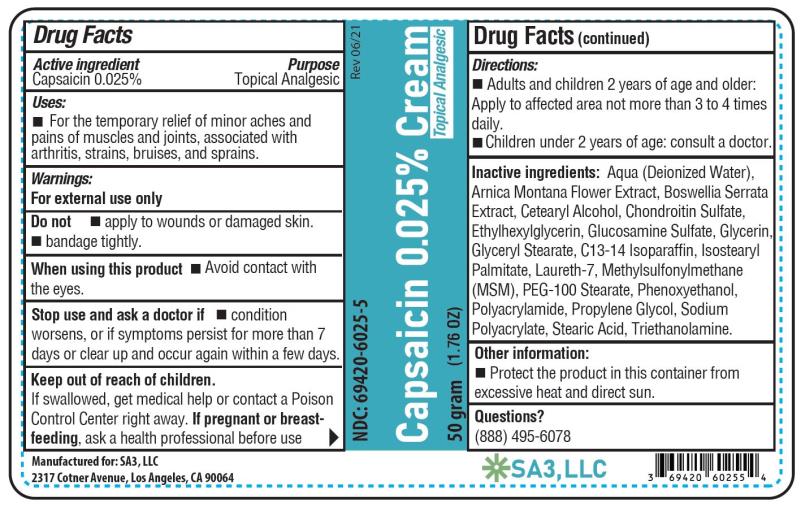
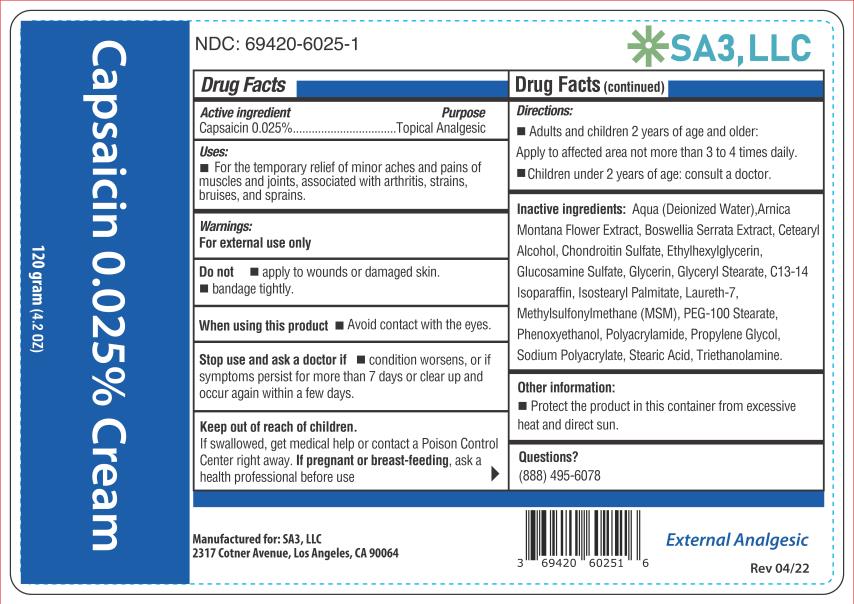
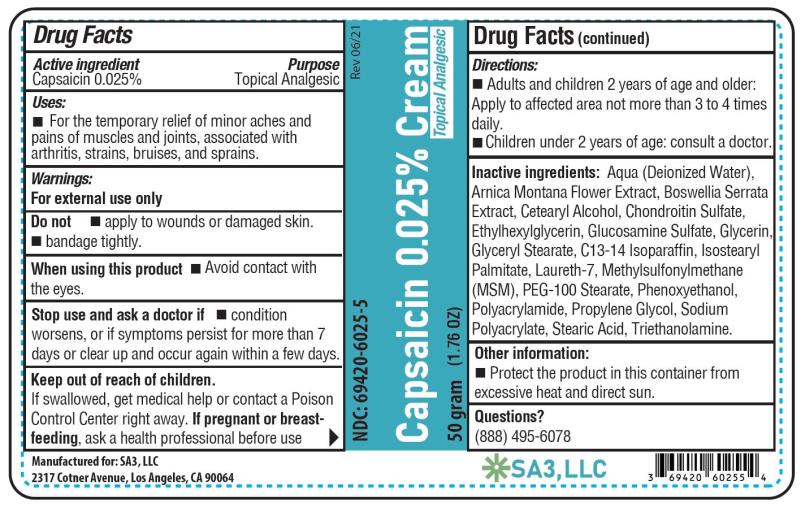
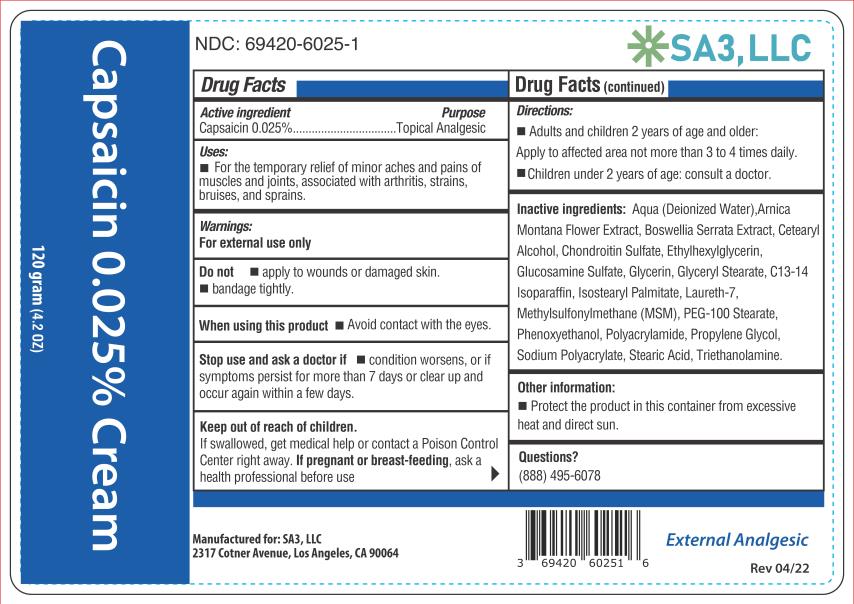
Active ingredient
Capsaicin 0.025%
-
Purpose
Topical analgesic
-
Uses
Temporarily relieves minor aches and pains of muscles and joints due to: • simple backache - • arthritis - • strains - • sprains
-
Warnings
For external use only - Read all warnings and directions before use. Test first on small area of skin. Do not - • Apply to wounds or damaged skin - • Bandage tightly - • If you are allergic to ...
-
Directions
Adults and children 2 years of age and older: Apply to affected area not more than 3 to 4 times daily. Wash hands thoroughly with soap and water immediately after application. Children under 2 ...
-
Other information
Store at room temperature 15°-30°C (59°-86°F). Protect the product from excessive heat and direct sun.
-
Inactive ingredients
Aqua (Deionized Water), Arnica Montana Flower Extract, Boswellia Serrata Extract, Cetearyl Alcohol, Chondroitin Sulfate, Ethylhexylglycerin, Glucosamine Sulfate, Glycerin, Glyceryl Stearate ...
-
PRINCIPAL DISPLAY PANEL
Capsaicin 0.025% cream - NDC 69420-6025-5 - 50 grams - SA3, LLC
-
PRINCIPAL DISPLAY PANEL
Capsaicin 0.025% cream - NDC 69420-6025-1 - 120 grams - SA3, LLC
-
INGREDIENTS AND APPEARANCEProduct Information