Label: PHENAZOPYRIDINE HYDROCHLORIDE tablet
- NDC Code(s): 69367-611-01, 69367-612-01
- Packager: Westminster Pharmaceuticals, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated October 31, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONRx only - Phenazopyridine Hydrochloride – Westminster Pharmaceuticals, LLC - Prescription Medication - Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has ...
-
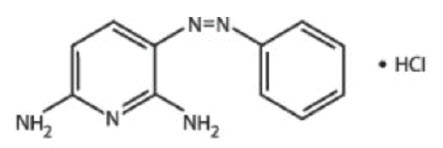
DESCRIPTIONPhenazopyridine Hydrochloride is a reddish-brown, odorless, slightly bitter, crystalline powder. It has a specific local analgesic effect in the urinary tract, promptly relieving burning and ...
-
CLINICAL PHARMACOLOGYPhenazopyridine hydrochloride is excreted in the urine where it exerts a topical analgesic effect on the mucosa of the urinary tract. This action helps to relieve pain, burning, urgency and ...
-
INDICATIONS AND USAGEPhenazopyridine Hydrochloride is indicated for the symptomatic relief of pain, burning, urgency frequency, and other discomforts arising from irritation of the mucosa of the lower urinary tract ...
-
CONTRAINDICATIONSPhenazopyridine Hydrochloride should not be used in patients who are hypersensitive to the drug or its ingredients. Phenazopyridine Hydrochloride is contraindicated in patients with renal ...
-
WARNINGSPhenazopyridine Hydrochloride is reasonably anticipated to be a human carcinogen based on sufficient evidence of carcinogenicity in experimental animals (IARC 1980, 1982, 1987, NCI 1978). When ...
-
PRECAUTIONSGeneral - The patient should be advised that Phenazopyridine Hydrochloride produces an orange to red color in the urine and feces, and may cause staining. Phenazopyridine Hydrochloride may cause ...
-
DOSAGE AND ADMINISTRATION100 mg Tablets: Average adult dosage is two tablets 3 times a day after meals. 200 mg Tablets: Average adult dosage is one tablet 3 times a day after meals. When used concomitantly with an ...
-
ADVERSE REACTIONSThe following adverse events have been reported: CNS: headache. Gastrointestinal: nausea, vomiting and diarrhea. Dermatologic and Hypersensitivity: rash, pruritus, discoloration ...
-
HOW SUPPLIED100 mg Tablets: Supplied in bottles of 100ct (NDC 69367-611-01) Appearance: Dark brown coated, round standard cup tablet, debossed "611" on one side and plain on the other. 200 mg Tablets ...
-
SPL UNCLASSIFIED SECTIONManufactured for: Westminster Pharmaceuticals, LLC - Nashville, TN 37217 - Rev: 08/2023
-
PRINCIPAL DISPLAY PANEL - 100 mg Tablet Bottle LabelNDC 69367-611-01 - Rx Only - Phenazopyridine - Hydrochloride - Tablets, USP - 100 mg - 100 Tablets - Westminster - Pharmaceuticals
-
PRINCIPAL DISPLAY PANEL - 200 mg Tablet Bottle LabelNDC 69367-612-01 - Rx Only - Phenazopyridine - Hydrochloride - Tablets, USP - 200 mg - 100 Tablets - Westminster - Pharmaceuticals
-
INGREDIENTS AND APPEARANCEProduct Information