Label: FELODIPINE tablet, extended release
FELOPDIPINE- felodipine tablet, extended release
- NDC Code(s): 69367-264-01, 69367-265-01, 69367-266-01
- Packager: Westminster Pharmaceuticals, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated April 4, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
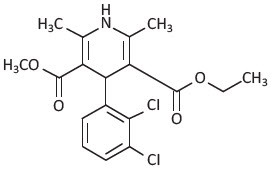
DESCRIPTIONFelodipine is a calcium antagonist (calcium channel blocker). Felodipine is a dihydropyridine derivative that is chemically described as ± ethyl methyl 4-(2,3-dichlorophenyl) -1,4-dihydro-2 ...
-
CLINICAL PHARMACOLOGYMechanism of Action - Felodipine is a member of the dihydropyridine class of calcium channel antagonists (calcium channel blockers). It reversibly competes with nitrendipine and/or other calcium ...
-
INDICATIONS AND USAGEFelodipine Extended-release Tablets, USP are indicated for the treatment of hypertension, to lower blood pressure. Lowering blood pressure lowers the risk of fatal and non-fatal cardiovascular ...
-
CONTRAINDICATIONSFelodipine is contraindicated in patients who are hypersensitive to this product.
-
PRECAUTIONSGeneral - Hypotension - Felodipine, like other calcium antagonists, may occasionally precipitate significant hypotension and, rarely, syncope. It may lead to reflex tachycardia which in ...
-
ADVERSE REACTIONSTo report SUSPECTED ADVERSE REACTIONS, contact Westminster Pharmaceuticals, LLC at 1-844-221-7294 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. In controlled studies in the United States and ...
-
OVERDOSAGEOral doses of 240 mg/kg and 264 mg/kg in male and female mice, respectively, and 2390 mg/kg and 2250 mg/kg in male and female rats, respectively, caused significant lethality. In a suicide ...
-
DOSAGE AND ADMINISTRATIONThe recommended starting dose is 5 mg once a day. Depending on the patient's response, the dosage can be decreased to 2.5 mg or increased to 10 mg once a day. These adjustments should occur ...
-
HOW SUPPLIEDFelodipine Extended-release Tablets, USP 2.5 mg, are yellow film-coated, round convex tablets, with code Y161 on one side. They are supplied as follows: NDC 69367-264-01 bottles of ...
-
SPL UNCLASSIFIED SECTIONManufactured by: Yiling Pharmaceutical Ltd - No. 36 Zhujiang Road, Shijiazhuang, Hebei, 050035, China - Distributed by: Westminster Pharmaceuticals, LLC - 1321 Murfreesboro Pike, Ste 607, Nashville, TN ...
-
PRINCIPAL DISPLAY PANEL - 2.5 mg Tablet Bottle LabelNDC 69367-264-01 - RX Only - Felodipine - Extended-Release - Tablets, USP - 2.5 mg - 100 Tablets - Westminster - Pharmaceuticals
-
PRINCIPAL DISPLAY PANEL - 5 mg Tablet Bottle LabelNDC 69367-265-01 - RX Only - Felodipine - Extended-Release - Tablets, USP - 5 mg - 100 Tablets - Westminster - Pharmaceuticals
-
PRINCIPAL DISPLAY PANEL - 10 mg Tablet Bottle LabelNDC 69367-266-01 - RX Only - Felodipine - Extended-Release - Tablets, USP - 10 mg - 100 Tablets - Westminster - Pharmaceuticals
-
INGREDIENTS AND APPEARANCEProduct Information