Label: SODIUM SULFACETAMIDE, SULFUR- sulfacetamide sodium and sulfur liquid
- NDC Code(s): 69367-247-06, 69367-247-12
- Packager: Westminster Pharmaceuticals, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated May 27, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONRx Only - FOR EXTERNAL USE ONLY. NOT FOR OPHTHALMIC USE.
-
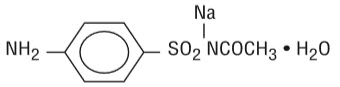
DESCRIPTIONEach gram of Sodium Sulfacetamide 10% and Sulfur 5% Cleanser contains 100 mg of sodium sulfacetamide and 50 mg of sulfur in a formulation containing ammonium lauryl sulfate, butylated ...
-
CLINICAL PHARMACOLOGYThe most widely accepted mechanism of action of sulfonamides is the Woods-Fildes theory, which is based on the fact that sulfonamides act as competitive antagonists to para-aminobenzoic acid ...
-
INDICATIONSSodium Sulfacetamide 10% and Sulfur 5% Cleanser is indicated in the topical control of acne vulgaris, acne rosacea and seborrheic dermatitis.
-
CONTRAINDICATIONSSodium Sulfacetamide 10% and Sulfur 5% Cleanser is contraindicated for use by patients having known hypersensitivity to sulfonamides, sulfur or any other component of this preparation. Sodium ...
-
WARNINGSAlthough rare, sensitivity to sodium sulfacetamide may occur. Therefore, caution and careful supervision should be observed when prescribing this drug for patients who may be prone to ...
-
PRECAUTIONSGeneral - If irritation develops, use of the product should be discontinued and appropriate therapy instituted. Patients should be carefully observed for possible local irritation or ...
-
ADVERSE REACTIONSAlthough rare, sodium sulfacetamide may cause local irritation. Call your doctor for medical advice about side effects. To report a serious adverse event, please contact Westminster ...
-
DOSAGE AND ADMINISTRATIONApply Sodium Sulfacetamide 10% and Sulfur 5% Cleanser once or twice daily to affected areas, or as directed by your physician. Wet skin and liberally apply to areas to be cleansed. Massage gently ...
-
HOW SUPPLIEDSodium Sulfacetamide 10% and Sulfur 5% Cleanser is available in 6 oz. (170.3 g) bottles, NDC 69367-247-06 and 12 oz. (340.2 g) bottles, NDC 69367-247-12. Store at 15°C to 30°C (59°F to 86°F) ...
-
SPL UNCLASSIFIED SECTIONKEEP THIS AND ALL MEDICATIONS OUT OF THE REACH OF CHILDREN - All prescription substitutions and/or recommendations using this product shall be made subject to state and federal statutes as ...
-
SPL UNCLASSIFIED SECTIONManufactured for: Westminster Pharmaceuticals, LLC - Nashville, TN 37217 - Rev. 05/20
-
PRINCIPAL DISPLAY PANEL - 170.3 g Bottle LabelNDC 69367-247-06 - Rx Only - Sodium - Sulfacetamide 10% and Sulfur 5% Cleanser - FOR EXTERNAL USE ONLY - 6 oz (170.3 g) Westminster - Pharmaceuticals
-
INGREDIENTS AND APPEARANCEProduct Information