Label: NITROGLYCERIN tablet
- NDC Code(s): 69339-173-01, 69339-174-01, 69339-174-02, 69339-174-41, view more
- Packager: Natco Pharma USA LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated July 20, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use NITROGLYCERIN SUBLINGUAL TABLETS safely and effectively. See full prescribing information for NITROGLYCERIN SUBLINGUAL TABLETS ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGENitroglycerin sublingual tablets are indicated for the acute relief of an attack or acute prophylaxis of angina pectoris due to coronary artery disease.
-
2 DOSAGE AND ADMINISTRATIONAdminister one tablet under the tongue or in the buccal pouch at the first sign of an acute anginal attack. Allow tablet to dissolve without swallowing. One additional tablet may be administered ...
-
3 DOSAGE FORMS AND STRENGTHSNitroglycerin sublingual tablets, USP are supplied as white, round, flat-faced tablets in three strengths: 0.3 mg (Coded with “V” on one side and “3” on the other) 0.4 mg (Coded with “V ...
-
4 CONTRAINDICATIONS4.1 PDE-5-Inhibitors and sGC-Stimulators - Do not use nitroglycerin sublingual tablets in patients who are taking PDE-5 Inhibitors, such as avanafil, sildenafil, tadalafil, vardenafil ...
-
5 WARNINGS AND PRECAUTIONS5.1 Tolerance - Excessive use may lead to the development of tolerance. Only the smallest dose required for effective relief of the acute angina attack should be used. A decrease in therapeutic ...
-
6 ADVERSE REACTIONSThe following adverse reactions are discussed in more detail elsewhere in the label: Hypotension [see Warnings and Precautions (5.2)] Headache [see Warnings and Precautions ...
-
7 DRUG INTERACTIONS7.1 PDE-5-Inhibitors and sGC-Stimulators - Nitroglycerin sublingual tablets are contraindicated in patients who are using a selective inhibitor of cyclic guanosine monophosphate (cGMP)-specific ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk summary - Limited published data on the use of nitroglycerin are insufficient to determine a drug associated risk of major birth defects or miscarriage. In animal ...
-
10 OVERDOSAGE10.1 Signs and Symptoms, Methemoglobinemia - Nitrate overdosage may result in: severe hypotension, persistent throbbing headache, vertigo, palpitation, visual disturbance, flushing and ...
-
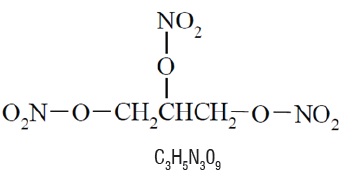
11 DESCRIPTIONNitroglycerin sublingual tablets USP, are a stabilized sublingual compressed nitroglycerin tablet that contains 0.3 mg, 0.4 mg, or 0.6 mg nitroglycerin. The sublingual tablets also contain the ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Nitroglycerin forms free radical nitric oxide (NO) which activates guanylate cyclase, resulting in an increase of guanosine 3’5’ monophosphate (cyclic GMP) in smooth ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis & Mutagenesis & Impairment Of Fertility - Animal carcinogenesis studies with sublingually administered nitroglycerin have not been performed. Carcinogenicity potential ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGNitroglycerin sublingual tablets, USP are supplied in 3 strengths in color-coded labeled bottles containing 100 tablets each (0.3 mg, 0.4 mg, and 0.6 mg), and in one strength in color-coded ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Patient Information). Product of USA - Manufactured by: NATCO PHARMA LIMITED - Kothur- 509 228, Telangana, India ...
-
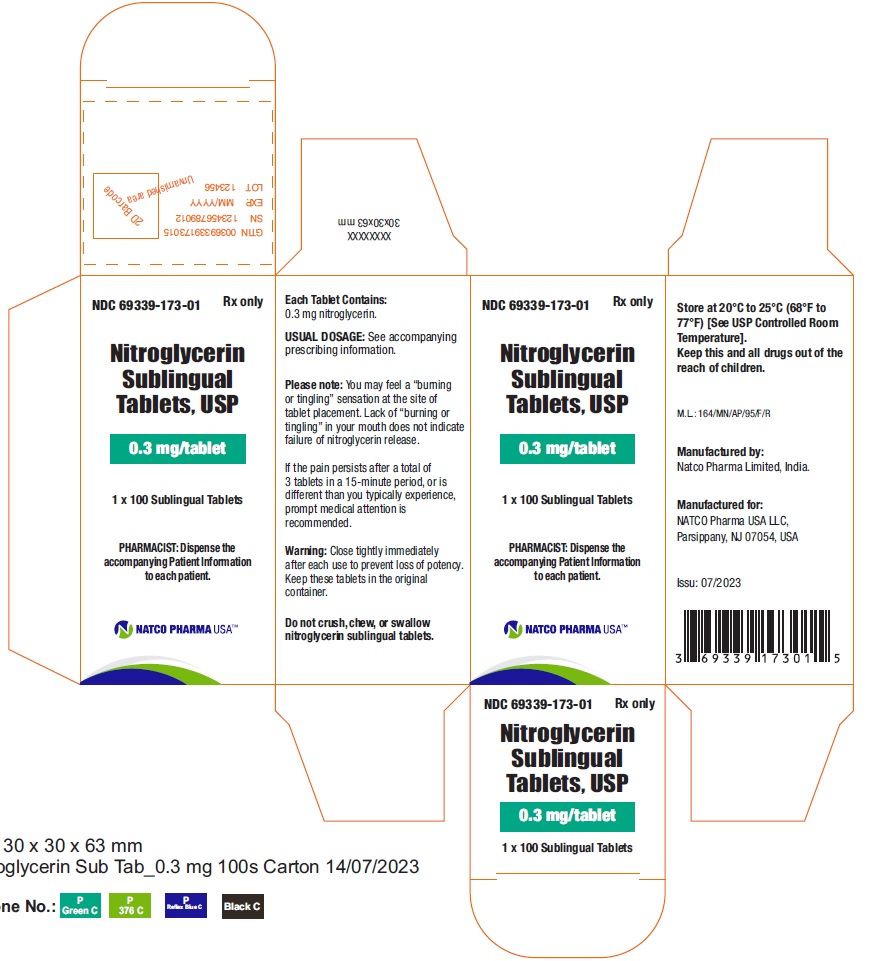
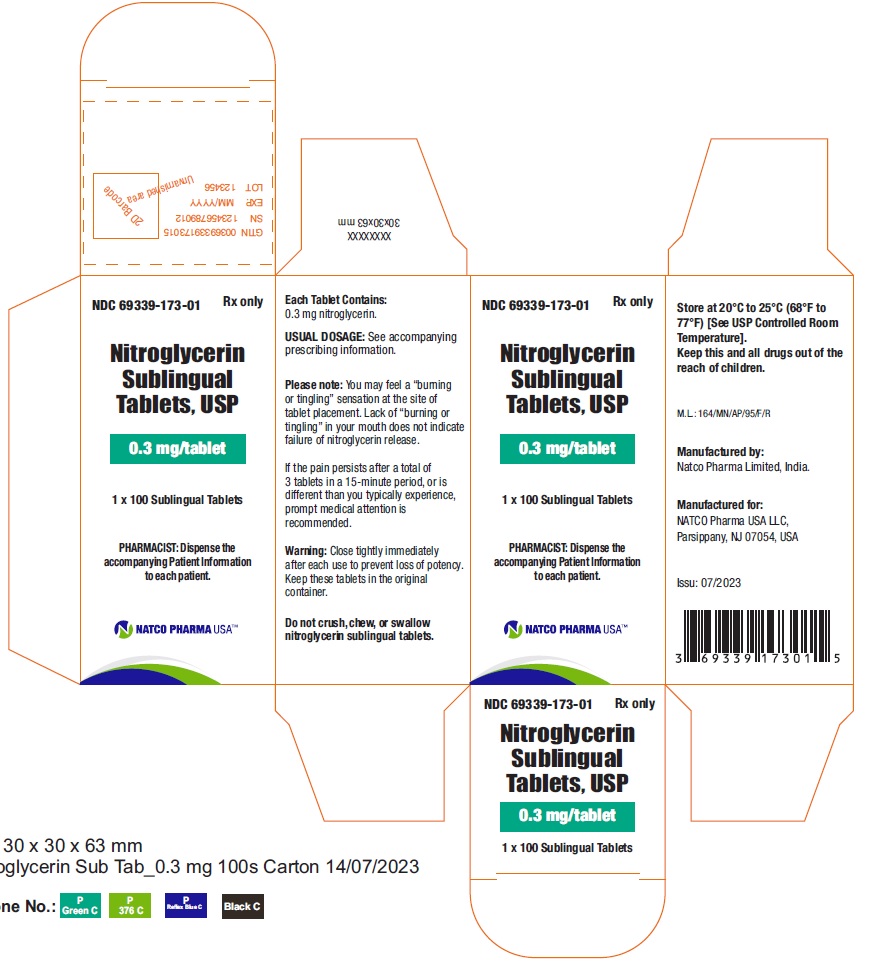
BOTTLE LABEL OF 0.3MG 100 TABLETSNDC 69339-173-01 - Nitroglycerin Sublingual Tablets, USP - 0.3 mg/tablet - PHARMACIST: Dispense the accompanying Patient Information to each patient. 1 X100 Sublingual Tablets - Rx ...
-
BOTTLE CARTON OF 0.3MG 100 TABLETSNDC 69339-173-01 - Nitroglycerin Sublingual Tablets, USP - 0.3 mg/tablet - PHARMACIST: Dispense the accompanying Patient Information to each patient. 1 x 100 Sublingual Tablets - Rx ...
-
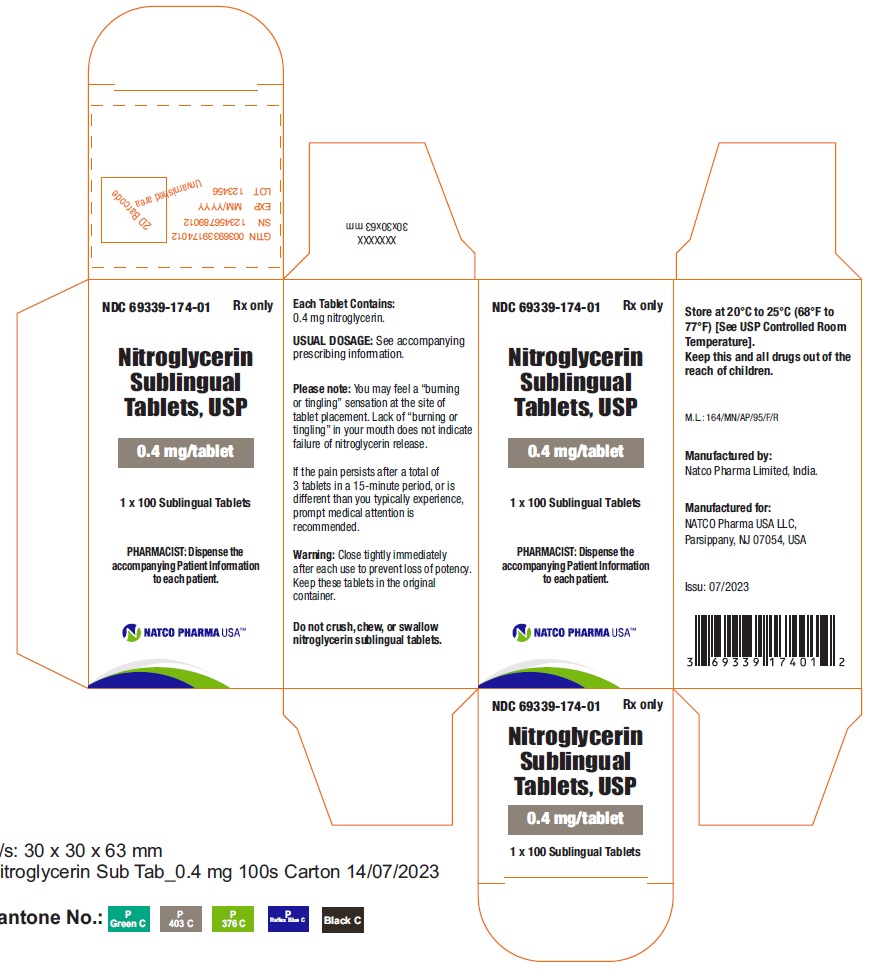
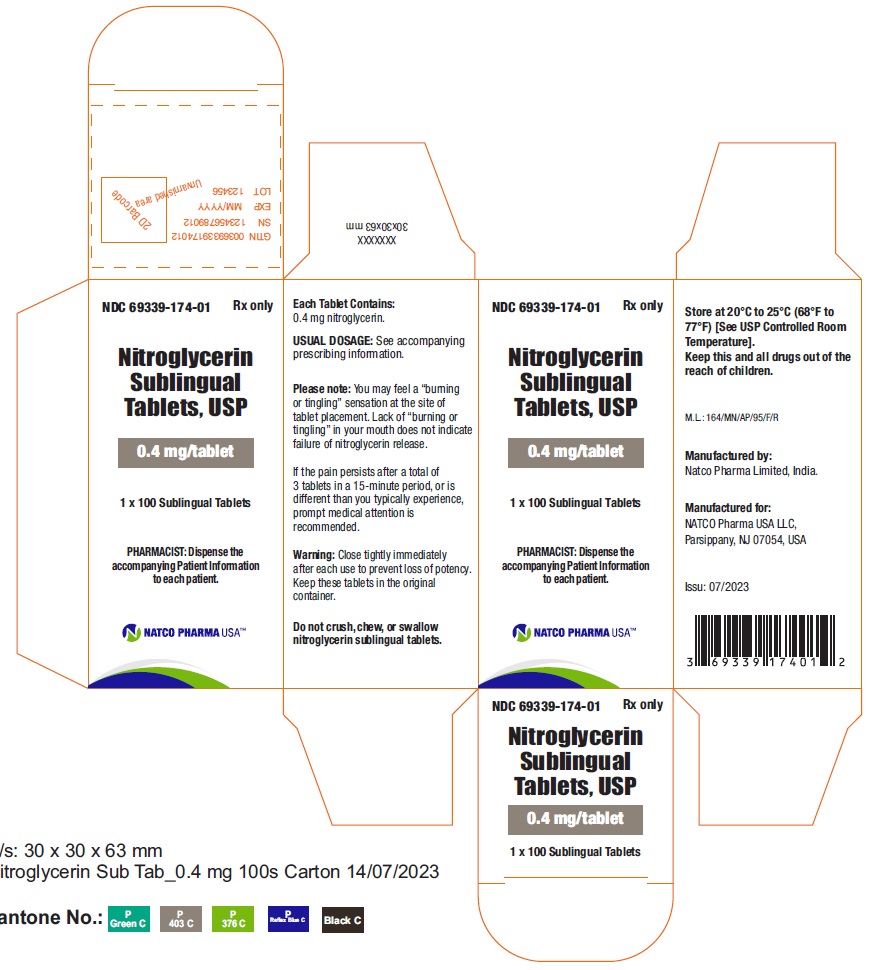
BOTTLE LABEL OF 0.4MG 100 TABELTSNDC 69339-174-01 - Nitroglycerin Sublingual Tablets, USP - 0.4 mg/tablet - PHARMACIST: Dispense the accompanying Patient Information to each patient. 1X100 Sublingual Tablets - Rx only
-
BOTTLE CARTON OF 0.4MG 100 TABLETSNDC 69339-174-01 - Nitroglycerin Sublingual Tablets, USP - 0.4 mg/tablet - PHARMACIST: Dispense the accompanying Patient Information to each patient. 1X100 Sublingual Tablets - Rx only
-
BOTTLE LABEL OF 0.4MG 4X25 TABLETSNDC 69339-174-02 - Nitroglycerin Sublingual Tablets, USP - 0.4 mg/tablet - PHARMACIST: Dispense the accompanying Patient Information to each patient. 4 x 25 Sublingual Tablets - Rx ...
-
BOTTLE CARTON OF 0.4MG 4X25 TABLETSNDC 69339-174-41 - Nitroglycerin Sublingual Tablets, USP - 0.4 mg/tablet - PHARMACIST: Dispense the accompanying Patient Information to each patient. 4 x 25 Sublingual Tablets - Rx only ...
-
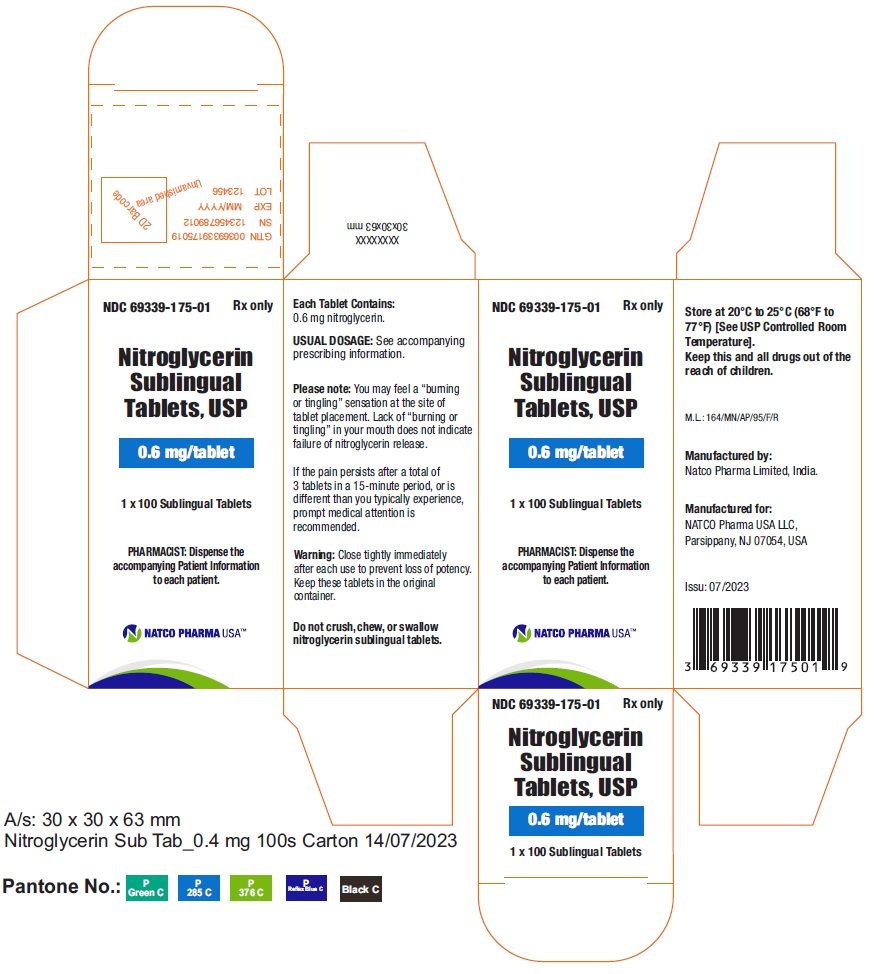
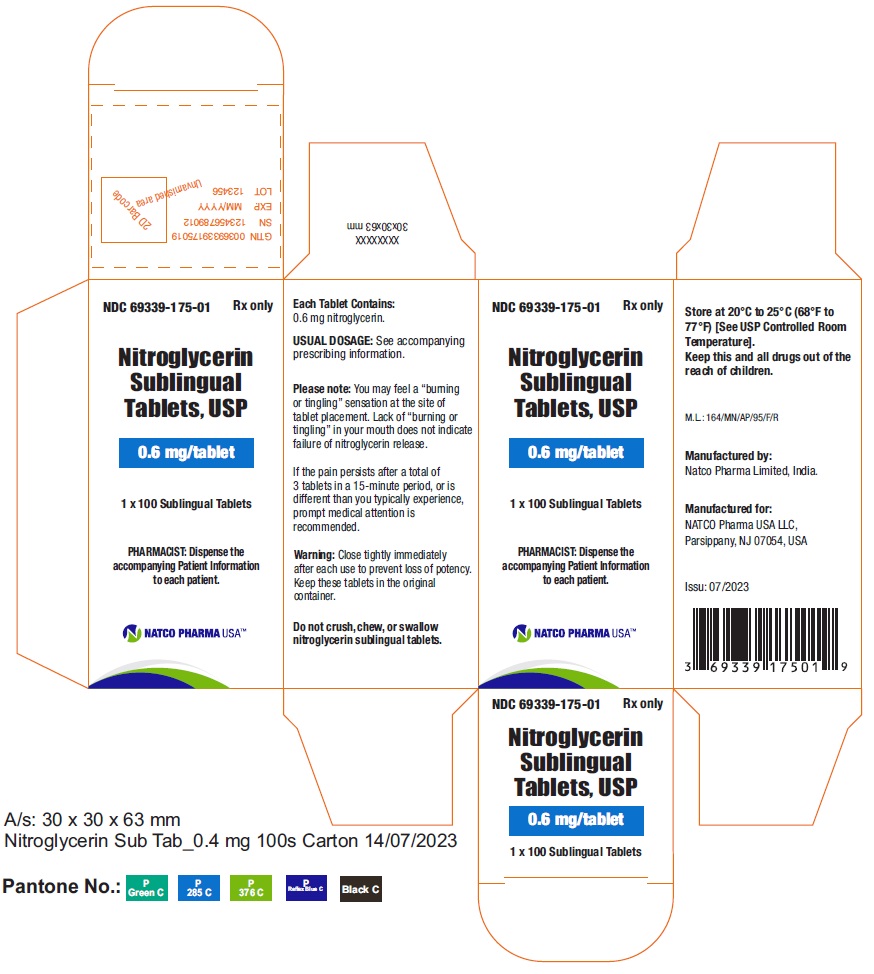
BOTTLE LABEL OF 0.6MG 100 TABLETSNDC 69339-175-01 - Nitroglycerin Sublingual Tablets, USP - 0.6 mg/tablet - PHARMACIST: Dispense the accompanying Patient Information to each patient. 1X100 Sublingual Tablets - Rx ...
-
BOTTLE CARTON OF 0.6MG 100 TABLETSNDC 69339-175-01 - Nitroglycerin Sublingual Tablets, USP - 0.6 mg/tablet - PHARMACIST: Dispense the accompanying Patient Information to each patient. 1 x 100 Sublingual Tablets - Rx only
-
INGREDIENTS AND APPEARANCEProduct Information