Label: TAVABOROLE solution
- NDC Code(s): 69238-1657-4, 69238-1657-6
- Packager: Amneal Pharmaceuticals NY LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated June 15, 2019
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use TAVABOROLE TOPICAL SOLUTION safely and effectively. See full prescribing information for TAVABOROLE TOPICAL SOLUTION. TAVABOROLE ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGETavaborole topical solution is an oxaborole antifungal indicated for the treatment of onychomycosis of the toenails due to Trichophyton rubrum or Trichophyton mentagrophytes.
-
2 DOSAGE AND ADMINISTRATIONApply tavaborole topical solution to affected toenails once daily for 48 weeks. Tavaborole topical solution should be applied to the entire toenail surface and under the tip of each toenail being ...
-
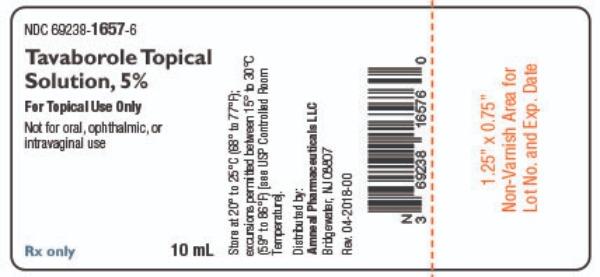
3 DOSAGE FORMS AND STRENGTHSTavaborole topical solution, 5% is a clear, colorless alcohol-based solution. Each milliliter of solution contains 43.5 mg (5% w/w) of tavaborole.
-
4 CONTRAINDICATIONSNone.
-
6 ADVERSE REACTIONS6.1 Clinical Trials Experience - Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - There are no available data on tavaborole use in pregnant women to inform a drug associated risk for major birth defects, miscarriage or adverse maternal or fetal ...
-
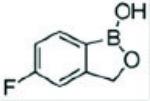
11 DESCRIPTIONTavaborole topical solution, 5% contains tavaborole, 5% (w/w) in a clear, colorless alcohol-based solution for topical use. The active ingredient, tavaborole, is an oxaborole antifungal with the ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Tavaborole is an oxaborole antifungal [see Clinical Pharmacology (12.4)]. 12.2 Pharmacodynamics - At therapeutic doses, tavaborole is not expected to prolong QTc to ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - In an oral carcinogenicity study in Sprague-Dawley rats, oral doses of 12.5, 25, and 50 mg/kg/day tavaborole were administered to rats ...
-
14 CLINICAL STUDIESThe efficacy and safety of tavaborole was evaluated in two multicenter, double-blind, randomized, vehicle-controlled trials. Tavaborole or vehicle was applied once daily for 48 weeks in subjects ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING16.1 How Supplied - Tavaborole Topical Solution, 5% is available as a clear, colorless solution free from visual foreign particulate matter filled in an amber glass bottle with a screw cap. At ...
-
17 PATIENT COUNSELING INFORMATIONSee FDA-approved patient labeling (Patient Information and Instructions for Use) The patient should be told the following: The impact of nail polish or other cosmetic nail products on the ...
-
PATIENT INFORMATION
Tavaborole (taʺ va borʹ ole) Topical Solution, 5% Important information: Tavaborole topical solution is for use on toenails only. Do not use tavaborole topical solution in your mouth ...
-
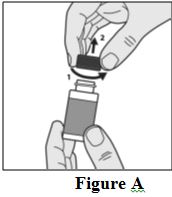
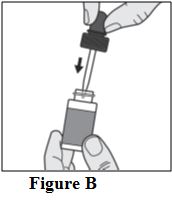
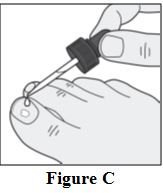
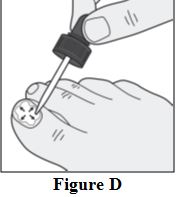
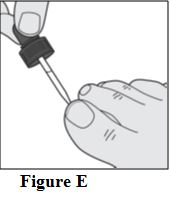
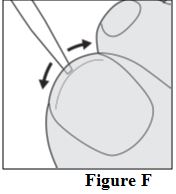
Instructions for Use
Tavaborole (taʺ va borʹ ole) Topical Solution, 5% Important information: Tavaborole topical solution is for use on toenails only. Do not use tavaborole topical solution in your mouth ...
-
PRINCIPAL DISPLAY PANEL
 ...
... -
INGREDIENTS AND APPEARANCEProduct Information