Label: CLOPIDOGREL BISULFATE tablet, film coated
-
Contains inactivated NDC Code(s)
NDC Code(s): 69117-1010-1, 69117-1010-2, 69117-1010-3, 69117-1010-4 - Packager: YILING PHARMACEUTICAL,INC.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated January 13, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use CLOPIDOGREL tablets, USP safely and effectively. See full prescribing information for CLOPIDOGREL tablets, USP. CLOPIDOGREL ...
-
Table of ContentsTable of Contents
-
BOXED WARNING
(What is this?)
WARNING: DIMINISHED ANTIPLATELET EFFECT IN PATIENTS WITH TWO LOSS-OF-FUNCTION ALLELES OF THE CYP2C19 GENE
The effectiveness of clopidogrel results from its antiplatelet activity, which is dependent on its conversion to an active metabolite by the cytochrome P450 (CYP) system, principally CYP2C19 [see Warnings and Precautions (5.1), Clinical Pharmacology (12.3)]. Clopdigorel at recommended doses forms less of the active metabolite and so has a reduced effect on platelet activity in patients who are homozygous for nonfunctional alleles of the CYP2C19 gene, (termed “CYP2C19 poor metabolizers”). Tests are available to identify patients who are CYP2C19 poor metabolizers [see Clinical Pharmacology (12.5)]. Consider use of another platelet P2Y12 inhibitor in patients identified as CYP2C19 poor metabolizers.
Close -
1 INDICATIONS AND USAGE1.1 Acute Coronary Syndrome (ACS) Clopidogrel is indicated to reduce the rate of myocardial infarction (MI) and stroke in patients with non-ST-segment elevation ACS [unstable angina ...
-
2 DOSAGE AND ADMINISTRATION2.1 Acute Coronary Syndrome - In patients who need an antiplatelet effect within hours, initiate clopidogrel with a single 300 mg oral loading dose and then continue at 75 mg once daily ...
-
3 DOSAGE FORMS AND STRENGTHSClopidogrel Tablets, USP 75 mg tablets: Pink colored, Round shaped, biconvex, film coated tablets de-bossed on one side with SG and 124 on other side. Clopidogrel Tablets, USP 300 mg tablets ...
-
4 CONTRAINDICATIONS4.1 Active Bleeding - Clopidogrel is contraindicated in patients with active pathological bleeding such as peptic ulcer or intracranial hemorrhage. 4.2 Hypersensitivity - Clopidogrel is ...
-
5 WARNINGS AND PRECAUTIONS5.1 Diminished Antiplatelet Activity in Patients with Impaired CYP2C19 Function - Clopidogrel is a prodrug. Inhibition of platelet aggregation by clopidogrel is achieved through an active ...
-
6 ADVERSE REACTIONSThe following serious adverse reactions are discussed below and elsewhere in the labeling: Bleeding - [see Warnings and Precautions (5.2)] Thrombotic thrombocytopenic purpura - [see ...
-
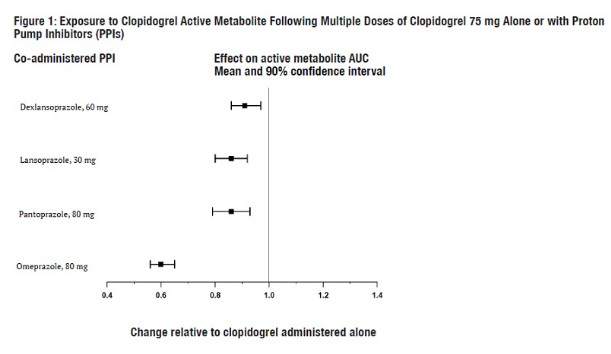
7 DRUG INTERACTIONS7.1 CYP2C19 Inhibitors - Clopidogrel is metabolized to its active metabolite in part by CYP2C19. Concomitant use of drugs that inhibit the activity of this enzyme results in reduced plasma ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Available data from cases reported in published literature and postmarketing surveillance with clopidogrel use in pregnant women have not identified any ...
-
10 OVERDOSAGEPlatelet inhibition by clopidogrel is irreversible and will last for the life of the platelet. Overdose following clopidogrel administration may result in bleeding complications. A single oral ...
-
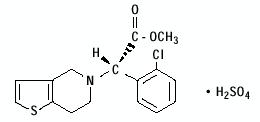
11 DESCRIPTIONClopidogrel bisulfate is a thienopyridine class inhibitor of P2Y - 12 ADP platelet receptors. Chemically it is methyl (+)-( S)-α-(2-chlorophenyl)-6,7-dihydrothieno[3,2-c]pyridine-5(4 ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Clopidogrel is an inhibitor of platelet activation and aggregation through the irreversible binding of its active metabolite to the P2Y - 12 class of ADP receptors on ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - There was no evidence of tumorigenicity when clopidogrel was administered for 78 weeks to mice and 104 weeks to rats at dosages up to 77 ...
-
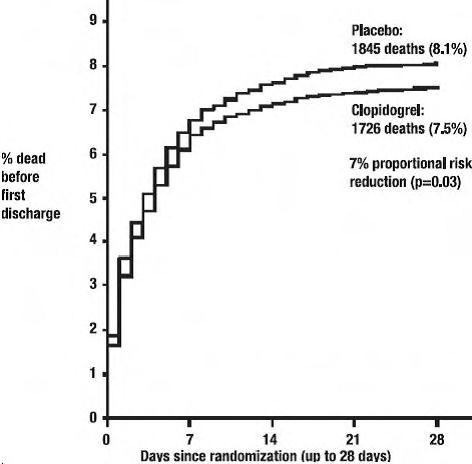
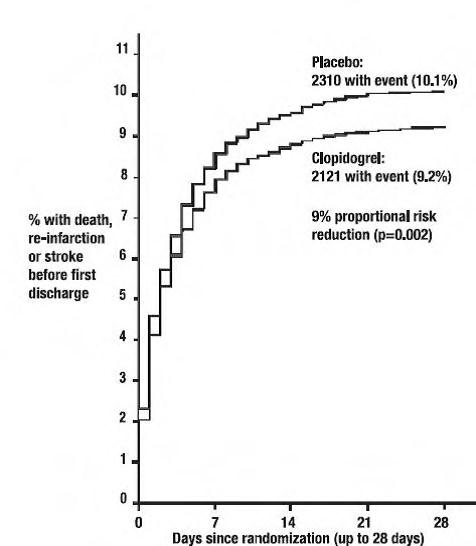
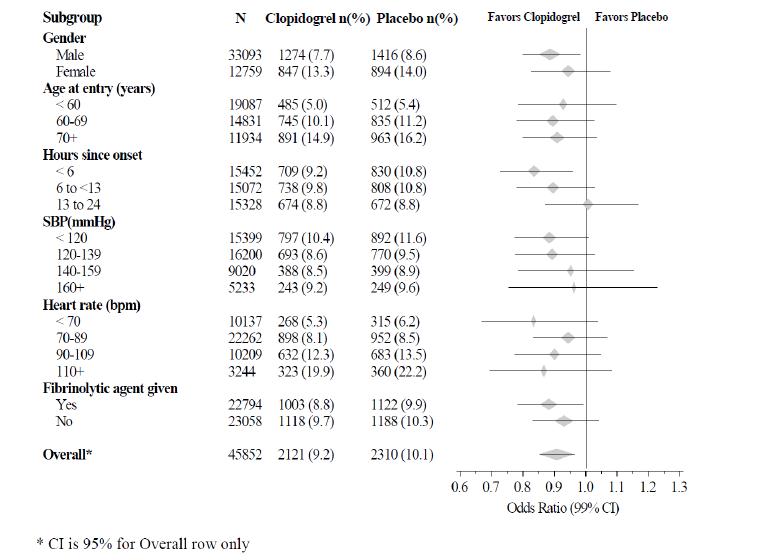
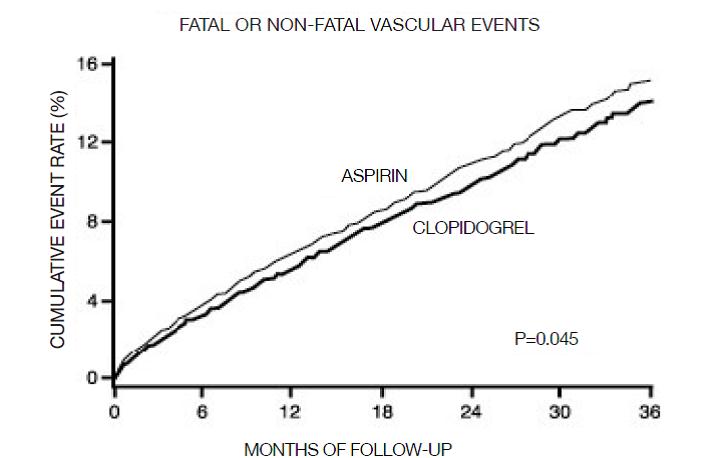
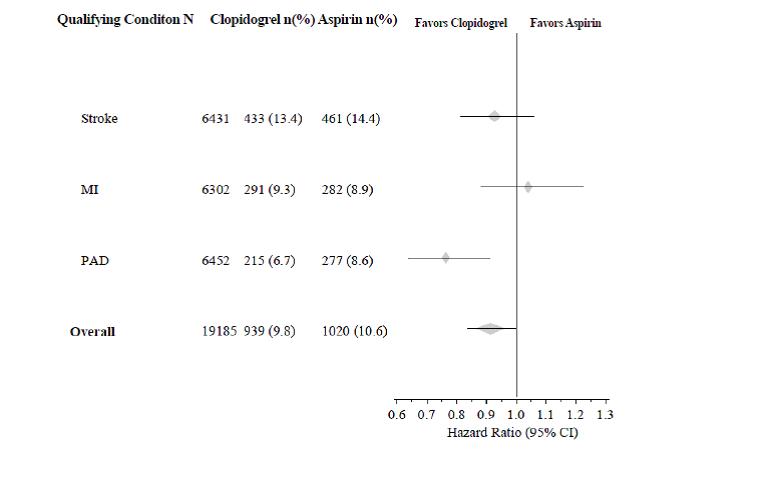
14 CLINICAL STUDIES14.1 Acute Coronary Syndrome - CURE - The CURE study included 12,562 patients with ACS without ST-elevation (UA or NSTEMI) and presenting within 24 hours of onset of the most recent episode of ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGClopidogrel tablets, USP 75 mg are available as pink colored, round shaped, biconvex, film coated tablets de-bossed on one side with SG and 124 on other side. Tablets are provided as follows: NDC ...
-
17 PATIENT COUNSELING INFORMATIONAdvise patients to read FDA approved patient labeling (Medication Guide). Discontinuation - Advise patients not to discontinue clopidogrel without first discussing it with the healthcare ...
-
Medication GuideClopidogrel Tablets, USP - (kloe pid' oh grel) Read this Medication Guide before you start taking clopidogrel tablets and each time you get a refill. There may be new information. This Medication ...
-
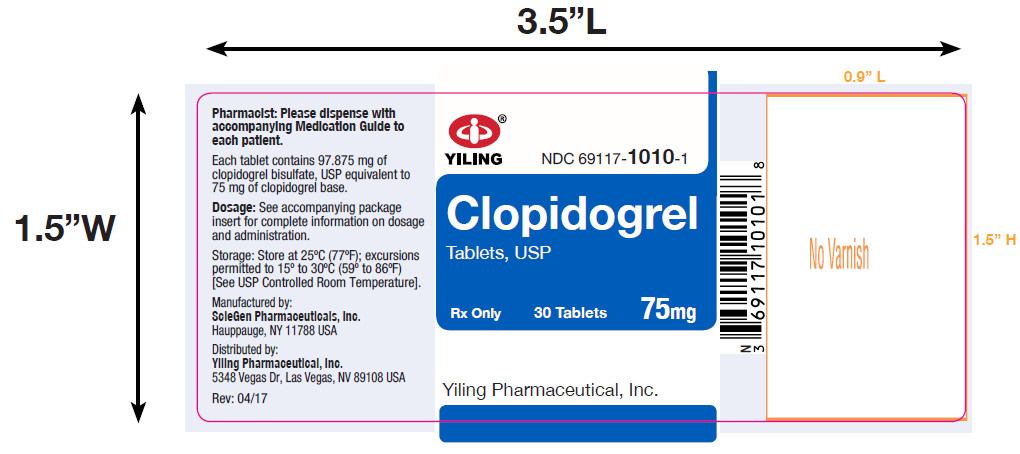
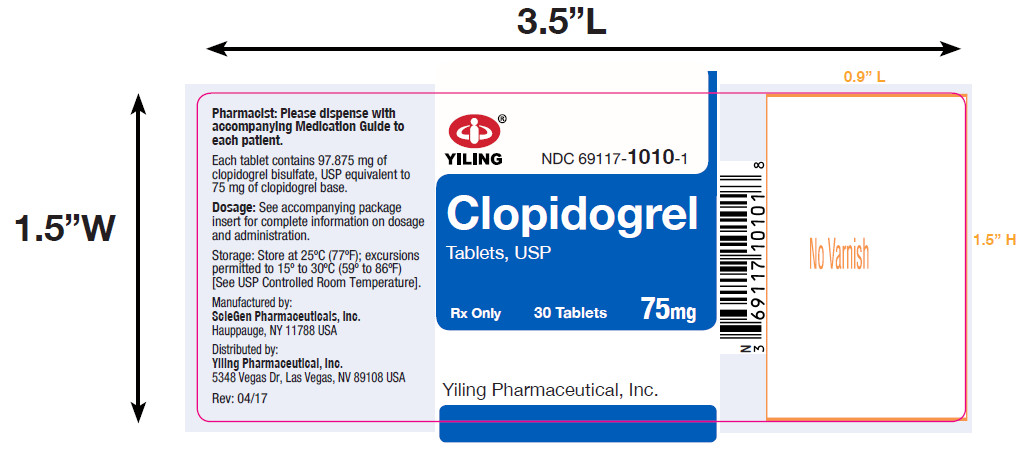
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL-75MG(30 TABLET BOTTLE)NDC 69117-1010-1 - CLOPIDOGREL - TABLETS,USP - 75 mg - PHARMACIST: Dispense the accompanying Medication Guide to each patient. 30 Tablets Rx only - Yiling Pharmaceutical, Inc.
-
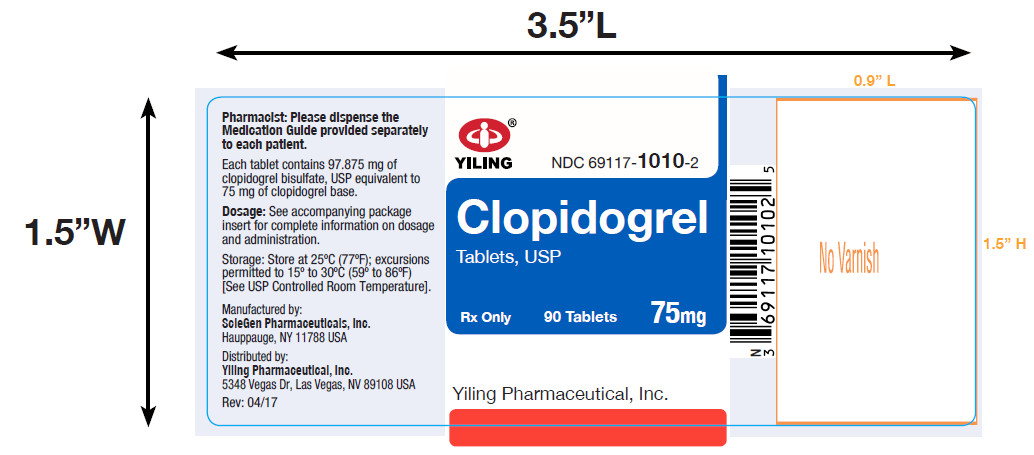
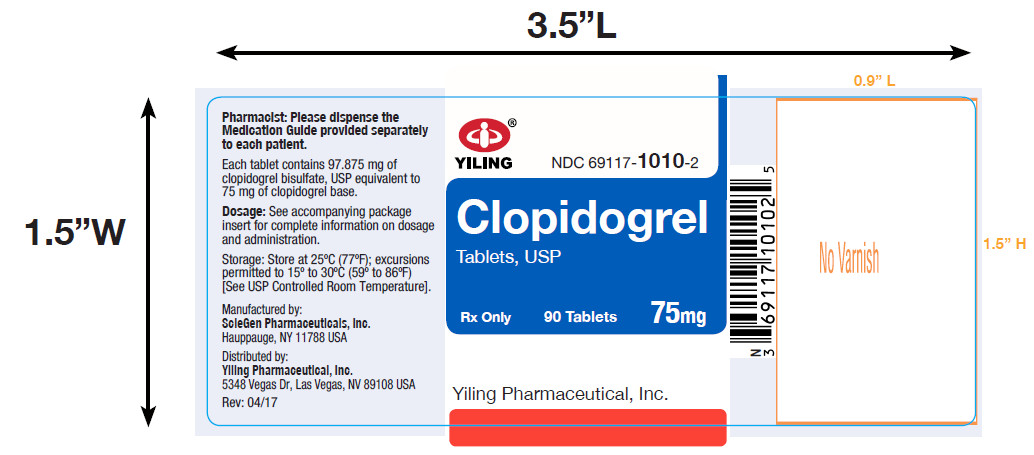
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL-75MG(90 TABLET BOTTLE)NDC 69117-1010-2 - CLOPIDOGREL - TABLETS,USP - 75 mg - PHARMACIST: Dispense the accompanying Medication Guide to each patient. 90 Tablets Rx only - Yiling Pharmaceutical, Inc
-
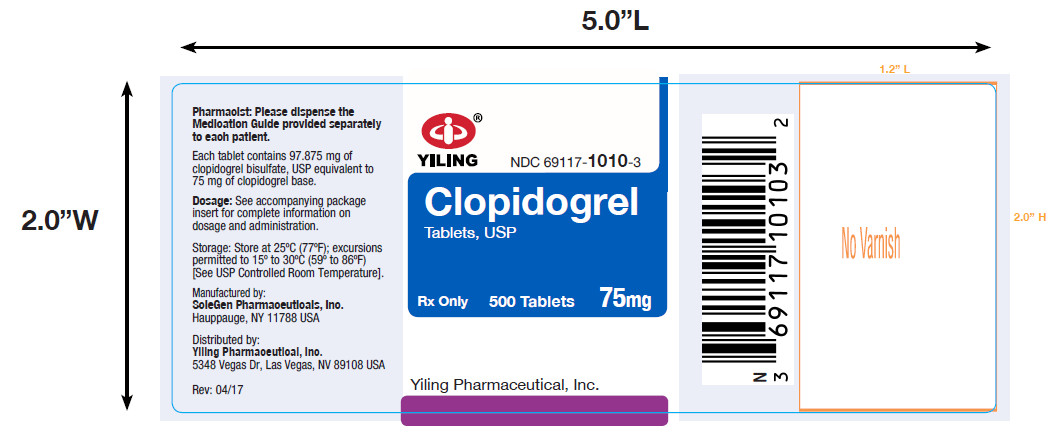
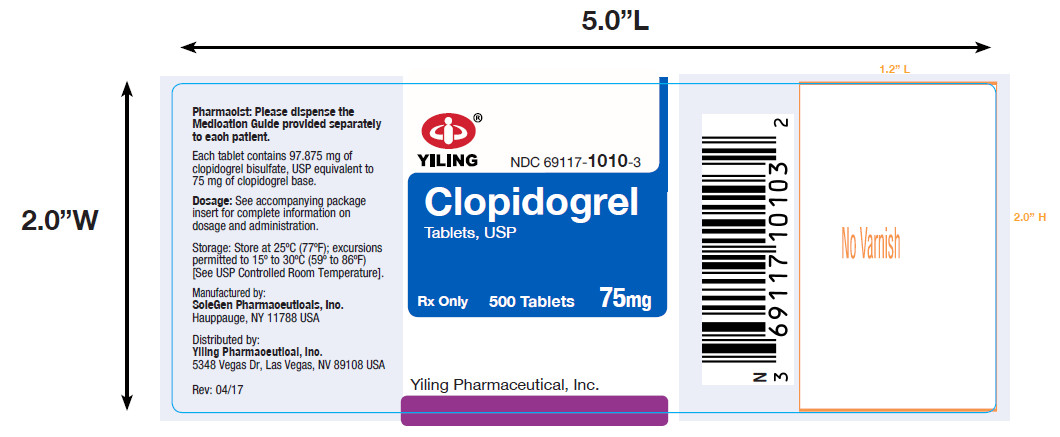
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL-75MG(500 TABLET BOTTLE)NDC 69117-1010-3 - CLOPIDOGREL - TABLETS,USP - 75 mg - PHARMACIST: Dispense the accompanying Medication Guide to each patient. 500 Tablets Rx only - Yiling Pharmaceutical, Inc.
-
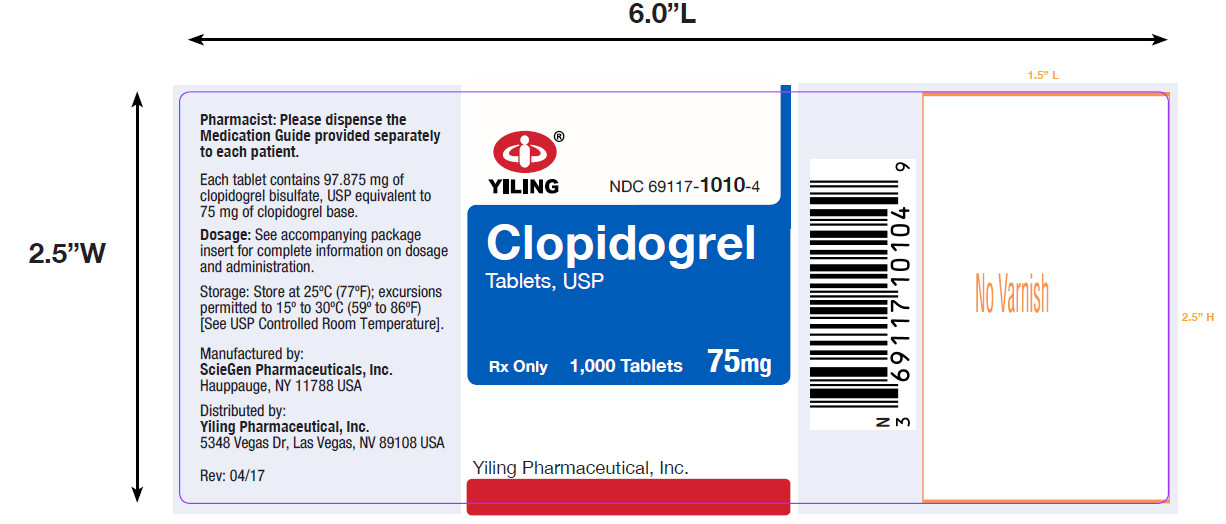
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL-75MG(1000 TABLET BOTTLE)NDC 69117-1010-4 - CLOPIDOGREL - TABLETS,USP - 75 mg - PHARMACIST: Dispense the accompanying Medication Guide to each patient. 1000 Tablets Rx only - Yiling Pharmaceutical, Inc. .
-
INGREDIENTS AND APPEARANCEProduct Information

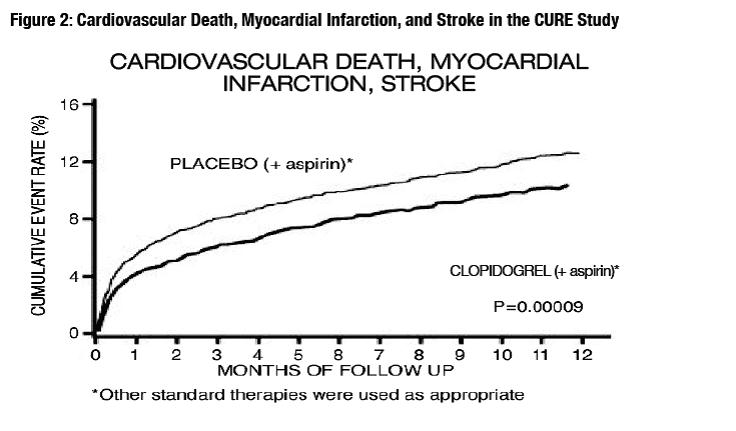
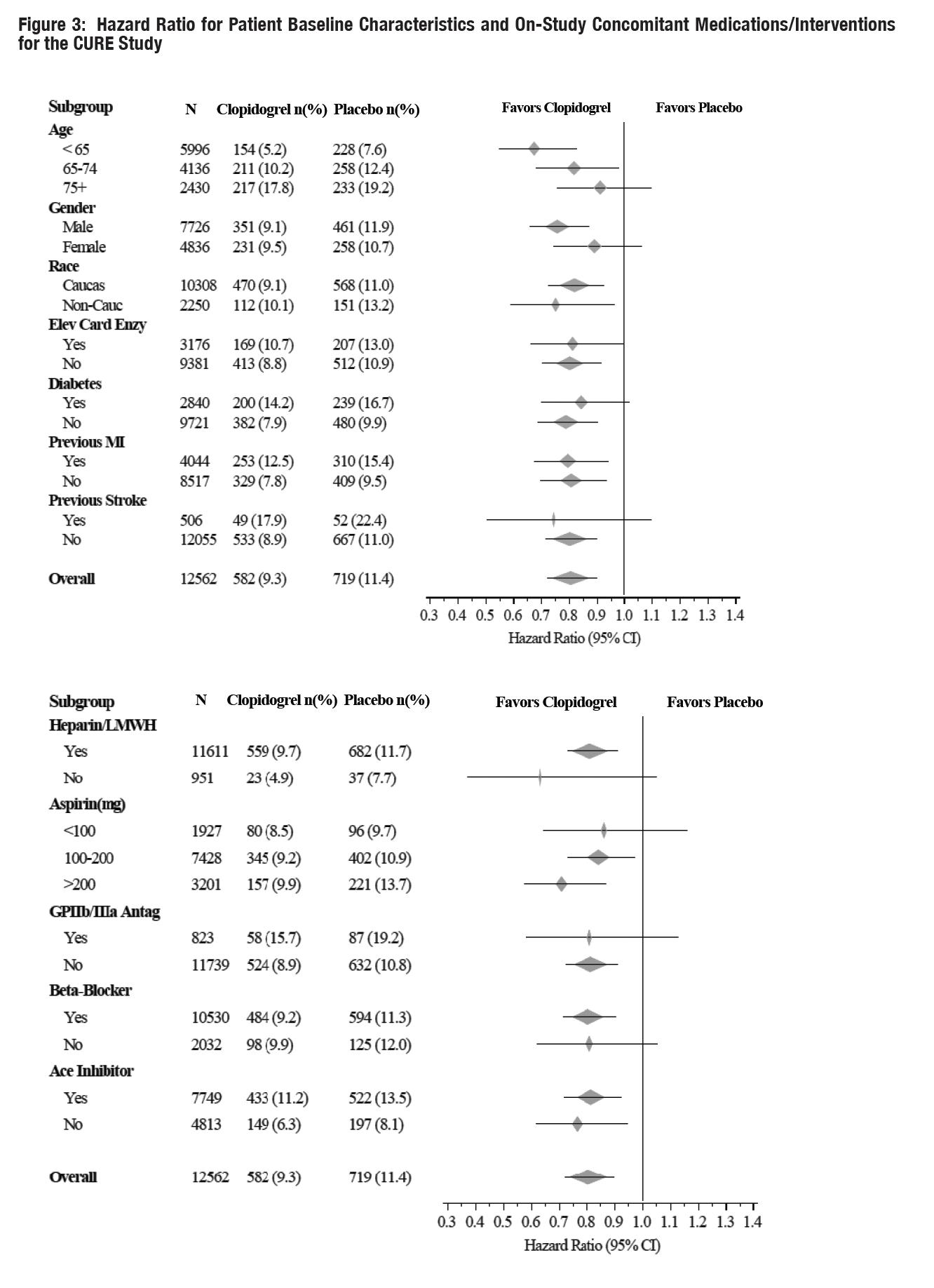 The use of clopidogrel in CURE was associated with a decrease in the use of thrombolytic therapy (71 patients [1.1%] in the clopidogrel group, 126 patients [2.0%] in the placebo group; relative risk reduction of 43%), and GPIIb/IIIa inhibitors (369 patients [5.9%] in the clopidogrel group, 454 patients [7.2%] in the placebo group, relative risk reduction of 18%). The use of clopidogrel in CURE did not affect the number of patients treated with CABG or PCI (with or without stenting), (2253 patients [36.0%] in the clopidogrel group, 2324 patients [36.9%] in the placebo group; relative risk reduction of 4.0%).
The use of clopidogrel in CURE was associated with a decrease in the use of thrombolytic therapy (71 patients [1.1%] in the clopidogrel group, 126 patients [2.0%] in the placebo group; relative risk reduction of 43%), and GPIIb/IIIa inhibitors (369 patients [5.9%] in the clopidogrel group, 454 patients [7.2%] in the placebo group, relative risk reduction of 18%). The use of clopidogrel in CURE did not affect the number of patients treated with CABG or PCI (with or without stenting), (2253 patients [36.0%] in the clopidogrel group, 2324 patients [36.9%] in the placebo group; relative risk reduction of 4.0%).