Label: RIZATRIPTAN BENZOATE tablet
- NDC Code(s): 69097-865-17, 69097-865-85, 69097-866-17, 69097-866-85
- Packager: Cipla USA Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated January 13, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use rizatriptan benzoate tablets safely and effectively. See full prescribing information for rizatriptan benzoate tablets. Rizatriptan ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGERizatriptan benzoate tablets are indicated for the acute treatment of migraine with or without aura in adults and in pediatric patients 6 to 17 years old. Limitations of Use - Rizatriptan ...
-
2 DOSAGE AND ADMINISTRATION2.1 Dosing Information in Adults - The recommended starting dose of rizatriptan benzoate tablets is either 5 mg or 10 mg for the acute treatment of migraines in adults. The 10 mg dose may provide ...
-
3 DOSAGE FORMS AND STRENGTHSRizatriptan benzoate tablets, USP: 5 mg tablets are pink, oval-shaped tablets debossed with '462' on one side and "IG" on the other side. 10 mg tablets are pink, oval-shaped ...
-
4 CONTRAINDICATIONSRizatriptan benzoate tablets are contraindicated in patients with: Ischemic coronary artery disease (angina pectoris, history of myocardial infarction, or documented silent ischemia), or other ...
-
5 WARNINGS AND PRECAUTIONS5.1 Myocardial Ischemia, Myocardial Infarction, and Prinzmetal's Angina - Rizatriptan benzoate should not be given to patients with ischemic or vasospastic coronary artery disease. There have ...
-
6 ADVERSE REACTIONSThe following adverse reactions are discussed in more detail in other sections of the labeling: Myocardial Ischemia, Myocardial Infarction, and Prinzmetal's Angina [see Warnings and ...
-
7 DRUG INTERACTIONS7.1 Propranolol - The dose of rizatriptan benzoate should be adjusted in propranolol-treated patients, as propranolol has been shown to increase the plasma AUC of rizatriptan by 70% [see Dosage ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Available human data on the use of rizatriptan in pregnant women are not sufficient to draw conclusions about drug-associated risk for major birth defects and ...
-
10 OVERDOSAGENo overdoses of rizatriptan benzoate were reported during clinical trials in adults. Some adult patients who received 40 mg of rizatriptan benzoate either a single dose or as two doses with a ...
-
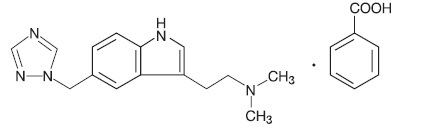
11 DESCRIPTIONRizatriptan benzoate tablets, USP contain rizatriptan benzoate, a selective 5-hydroxytryptamine1B/1D (5-HT1B/1D) receptor agonist. Rizatriptan benzoate, USP is described chemically as ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Rizatriptan binds with high affinity to human cloned 5-HT1B/1D receptors. Rizatriptan presumably exerts its therapeutic effects in the treatment of migraine headache by ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis: Oral carcinogenicity studies were conducted in mice (100 weeks) and rats (106 weeks) at doses of up to 125 mg/ kg/day ...
-
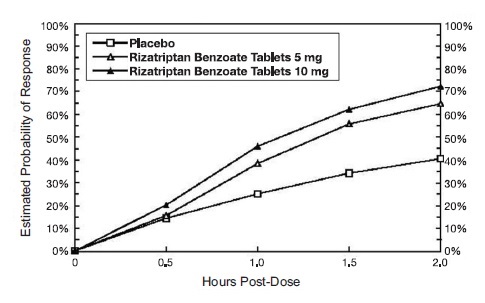
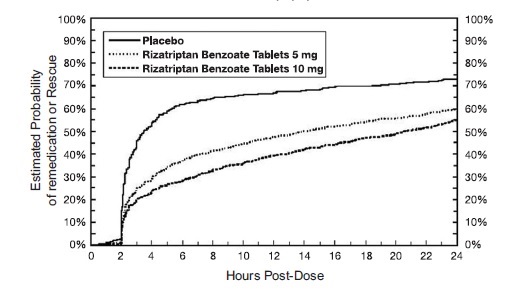
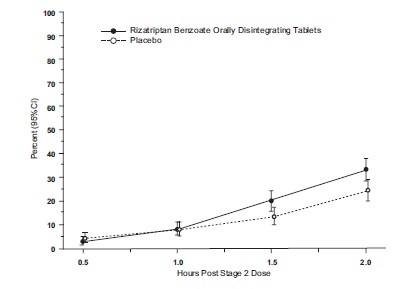
14 CLINICAL STUDIES14.1 Adults - The efficacy of rizatriptan benzoate tablets was established in four multicenter, randomized, placebo-controlled trials. Patients enrolled in these studies were primarily female ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGRizatriptan benzoate tablets, USP 5 mg, are pink, oval-shaped tablets debossed with '462'on one side and "IG" on the other side: NDC 69097-865-17, unit-of-use blister pack of 6 tablets; available ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-Approved Patient Labeling (Patient Information). Risk of Myocardial Ischemia and/or Infarction, Prinzmetal's Angina, Other Vasospasm-related Events, and ...
-
PATIENT INFORMATIONRIZATRIPTAN BENZOATE TABLETS, USP - (rye'' za trip' tan ben' zoe ate). 5 mg and 10 mg - Read this Patient Information before you start taking rizatriptan benzoate tablets and each time you get ...
- PATIENT PACKAGE INSERT
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANELNDC 69097-865-17 - Rizatriptan - Benzoate Tablets, USP - 5 mg (base) Warning: As with all medication, keep out of the reach of children. Cipla USA, Inc. NDC ...
-
INGREDIENTS AND APPEARANCEProduct Information