Label: FOSINOPRIL SODIUM- fosinopril tablet
-
NDC Code(s):
69097-856-05,
69097-856-15,
69097-857-05,
69097-857-15, view more69097-858-05, 69097-858-15
- Packager: Cipla USA Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated January 20, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
BOXED WARNING
(What is this?)
WARNING: FETAL TOXICITY
- When pregnancy is detected, discontinue fosinopril sodium tablets as soon as possible.
- Drugs that act directly on the renin-angiotensin system can cause injury and death to the developing fetus. See WARNINGS: Fetal Toxicity
-
DESCRIPTIONFosinopril sodium tablet, USP is the sodium salt of fosinopril USP, the ester prodrug of an angiotensin converting enzyme (ACE) inhibitor, fosinoprilat. It contains a phosphinate group capable ...
Fosinopril sodium tablet, USP is the sodium salt of fosinopril USP, the ester prodrug of an angiotensin converting enzyme (ACE) inhibitor, fosinoprilat. It contains a phosphinate group capable of specific binding to the active site of angiotensin converting enzyme.
Fosinopril sodium, USP is designated chemically as: L-proline,4-cyclohexyl-1-[[[2-methyl-1-(1-oxopropoxy) propoxy](4-phenylbutyl)phosphinyl]acetyl]-, sodium salt, trans-. Fosinopril sodium, USP is a white to off-white crystalline powder. It is soluble in water (100 mg/mL), methanol, and ethanol and slightly soluble in hexane. Its structural formula is:
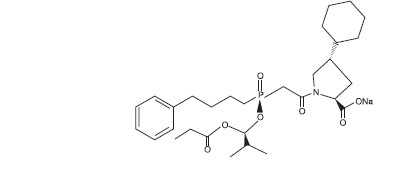
Its empirical formula is C30H45NNaO7P, and its molecular weight is 585.65.
Fosinopril Sodium, USP is available for oral administrations as 10 mg, 20 mg, and 40 mg tablets. Inactive ingredients include: crospovidone, lactose, microcrystalline cellulose, magnesium stearate, and povidone.
Close -
CLINICAL PHARMACOLOGYMechanism of Action - In animals and humans, fosinopril sodium is hydrolyzed by esterases to the pharmacologically active form, fosinoprilat, a specific competitive inhibitor of ...
Mechanism of Action
In animals and humans, fosinopril sodium is hydrolyzed by esterases to the pharmacologically active form, fosinoprilat, a specific competitive inhibitor of angiotensin-converting enzyme (ACE).
ACE is a peptidyl dipeptidase that catalyzes the conversion of angiotensin I to the vasoconstrictor substance, angiotensin II. Angiotensin II also stimulates aldosterone secretion by the adrenal cortex. Inhibition of ACE results in decreased plasma angiotensin II, which leads to decreased vasopressor activity and to decreased aldosterone secretion. The latter decrease may result in a small increase of serum potassium.
In 647 hypertensive patients treated with fosinopril alone for an average of 29 weeks, mean increases in serum potassium of 0.1 mEq/L were observed. Similar increases were observed among all patients treated with fosinopril, including those receiving concomitant diuretic therapy. Removal of angiotensin II negative feedback on renin secretion leads to increased plasma renin activity.
ACE is identical to kininase, an enzyme that degrades bradykinin. Whether increased levels of bradykinin, a potent vasodepressor peptide, play a role in the therapeutic effects of fosinopril sodium remains to be elucidated.
While the mechanism through which fosinopril sodium lowers blood pressure is believed to be primarily suppression of the renin-angiotensin-aldosterone system, fosinopril sodium has an antihypertensive effect even in patients with low-renin hypertension. Although fosinopril sodium was antihypertensive in all races studied, black hypertensive patients (usually a low-renin hypertensive population) had a smaller average response to ACE inhibitor monotherapy than non-black patients.
In patients with heart failure, the beneficial effects of fosinopril sodium are thought to result primarily from suppression of the renin-angiotensin-aldosterone system; inhibition of the angiotensin-converting enzyme produces decreases in both preload and afterload.
Pharmacokinetics and Metabolism
Following oral administration, fosinopril (the prodrug) is absorbed slowly. The absolute absorption of fosinopril averaged 36% of an oral dose. The primary site of absorption is the proximal small intestine (duodenum/jejunum). While the rate of absorption may be slowed by the presence of food in the gastrointestinal tract, the extent of absorption of fosinopril is essentially unaffected.
Fosinoprilat is highly protein-bound (approximately 99.4%), has a relatively small volume of distribution, and has negligible binding to cellular components in blood. After single and multiple oral doses, plasma levels, areas under plasma concentration-time curves (AUCs) and peak concentrations (Cmaxs) are directly proportional to the dose of fosinopril. Times to peak concentrations are independent of dose and are achieved in approximately 3 hours.
After an oral dose of radiolabeled fosinopril, 75% of radioactivity in plasma was present as active fosinoprilat, 20% to 30% as a glucuronide conjugate of fosinoprilat, and 1% to 5% as a p-hydroxy metabolite of fosinoprilat. Since fosinoprilat is not biotransformed after intravenous administration, fosinopril, not fosinoprilat, appears to be the precursor for the glucuronide and p-hydroxy metabolites. In rats, the p-hydroxy metabolite of fosinoprilat is as potent an inhibitor of ACE as fosinoprilat; the glucuronide conjugate is devoid of ACE inhibitory activity.
After intravenous administration, fosinoprilat was eliminated approximately equally by the liver and kidney. After oral administration of radiolabeled fosinopril, approximately half of the absorbed dose is excreted in the urine and the remainder is excreted in the feces. In two studies involving healthy subjects, the mean body clearance of intravenous fosinoprilat was between 26 and 39 mL/min.
In healthy subjects, the terminal elimination half-life (t1/2) of an intravenous dose of radiolabeled fosinoprilat is approximately 12 hours. In hypertensive patients with normal renal and hepatic function, who received repeated doses of fosinopril, the effective t1/2 for accumulation of fosinoprilat averaged 11.5 hours. In patients with heart failure, the effective t1/2 was 14 hours.
In patients with mild to severe renal insufficiency (creatinine clearance 10 to 80 mL/min/1.73m2), the clearance of fosinoprilat does not differ appreciably from normal, because of the large contribution of hepatobiliary elimination. In patients with end-stage renal disease (creatinine clearance <10 mL/min/1.73m2), the total body clearance of fosinoprilat is approximately one-half of that in patients with normal renal function. (See DOSAGE AND ADMINISTRATION).
Fosinopril is not well dialyzed. Clearance of fosinoprilat by hemodialysis and peritoneal dialysis averages 2% and 7%, respectively, of urea clearances.
In patients with hepatic insufficiency (alcoholic or biliary cirrhosis), the extent of hydrolysis of fosinopril is not appreciably reduced, although the rate of hydrolysis may be slowed; the apparent total body clearance of fosinoprilat is approximately one-half of that in patients with normal hepatic function.
In elderly (male) subjects (65 to 74 years old) with clinically normal renal and hepatic function, there appear to be no significant differences in pharmacokinetic parameters for fosinoprilat compared to those of younger subjects (20 to 35 years old).
In pediatric patients, (N=20) age 6 to 16 years, with glomerular filtration rate ≥25 mL/min, given a single dose of fosinopril (0.3 mg/kg given as solution), the mean AUC and Cmax values of fosinoprilat (the active form of fosinopril) were similar to those seen in healthy adults receiving 20 mg (about 0.3 mg/kg for a 70 kg adult) of fosinopril as a solution. The terminal elimination half-life of fosinoprilat in pediatric patients was 11 to 13 hours, also similar to that observed in adults.
Fosinoprilat was found to cross the placenta of pregnant animals.
Studies in animals indicate that fosinopril and fosinoprilat do not cross the blood-brain barrier.
Pharmacodynamics and Clinical Effects
Serum ACE activity was inhibited by ≥90% at 2 to 12 hours after single doses of 10 to 40 mg of fosinopril. At 24 hours, serum ACE activity remained suppressed by 85%, 93%, and 93% in the 10, 20, and 40 mg dose groups, respectively.
Hypertension
Administration of fosinopril sodium tablets to patients with mild to moderate hypertension results in a reduction of both supine and standing blood pressure to about the same extent with no compensatory tachycardia. Symptomatic postural hypotension is infrequent, although it can occur in patients who are salt-and/or volume-depleted (see WARNINGS). Use of fosinopril sodium in combination with thiazide diuretics gives a blood pressure-lowering effect greater than that seen with either agent alone.
Following oral administration of single doses of 10 mg to 40 mg, fosinopril sodium lowered blood pressure within one hour, with peak reduction achieved 2 to 6 hours after dosing. The antihypertensive effect of a single dose persisted for 24 hours. Following four weeks of monotherapy in placebo-controlled trials in patients with mild to moderate hypertension, once daily doses of 20 mg to 80 mg lowered supine or seated systolic and diastolic blood pressures 24 hours after dosing by an average of 8 to 9/6 to 7 mmHg more than placebo. The trough effect was about 50% to 60% of the peak diastolic response and about 80% of the peak systolic response.
In most trials, the antihypertensive effect of fosinopril sodium increased during the first several weeks of repeated measurements. The antihypertensive effect of fosinopril sodium has been shown to continue during long-term therapy for at least 2 years. Abrupt withdrawal of fosinopril sodium has not resulted in a rapid increase in blood pressure.
Limited experience in controlled and uncontrolled trials combining fosinopril with a calcium channel blocker or a loop diuretic has indicated no unusual drug-drug interactions. Other ACE inhibitors have had less than additive effects with beta-adrenergic blockers, presumably because both drugs lower blood pressure by inhibiting parts of the renin-angiotensin system
ACE inhibitors are generally less effective in blacks than in non-blacks. The effectiveness of fosinopril sodium was not influenced by age, sex, or weight.
In hemodynamic studies in hypertensive patients, after three months of therapy, responses (changes in BP, heart rate, cardiac index, and PVR) to various stimuli (e.g., isometric exercise, 45° head-up tilt, and mental challenge) were unchanged compared to baseline, suggesting that fosinopril sodium does not affect the activity of the sympathetic nervous system. Reduction in systemic blood pressure appears to have been mediated by a decrease in peripheral vascular resistance without reflex cardiac effects. Similarly, renal, splanchnic, cerebral, and skeletal muscle blood flow were unchanged compared to baseline, as was glomerular filtration rate.
Pediatric
Reduction of blood pressure with low (0.1 mg/kg), medium (0.3 mg/kg) and high (0.6 mg/kg) target doses of once-daily fosinopril was evaluated in a randomized, double-blind study of 252 pediatric patients 6 to 16 years of age with hypertension or high-normal blood pressure. Fosinopril doses in the medium and high dose groups were titrated to target doses after one week and the total duration of treatment was four weeks. The maximum dose studied was 40 mg once daily. At the end of four weeks of treatment, the mean reductions from baseline in trough systolic blood pressure were similar in all three dose groups. Withdrawal of fosinopril treatment resulted in an increase in blood pressure towards baseline over a two week period. Fosinopril was generally well tolerated.
CloseHeart Failure
In a randomized, double-blind, placebo-controlled trial, 179 patients with heart failure, all receiving diuretics and some receiving digoxin, were administered single doses of 10,20, or 40 mg of fosinopril sodium or placebo. Doses of 20 and 40 mg of fosinopril sodium resulted in acute decreases in pulmonary capillary wedge pressure (preload) and mean arterial blood pressure and systemic vascular resistance (afterload). One hundred fifty-five of these patients were re-randomized to once daily therapy with fosinopril sodium (10, 20, or 40 mg) for an additional 10 weeks. Hemodynamic measurements made 24 hours after dosing showed (relative to baseline) continued reduction in pulmonary capillary wedge pressure, mean arterial blood pressure, right atrial pressure and an increase in cardiac index and stroke volume for the 20 mg and 40 mg dose groups. No tachyphylaxis was seen.
Fosinopril sodium was studied in 3 double-blind, placebo controlled, 12 to 24 week trials including a total of 734 patients with heart failure, with fosinopril sodium doses from 10 mg to 40 mg daily. Concomitant therapy in 2 of these 3 trials included diuretics and digitalis; in the third trial patients were receiving only diuretics. All 3 trials showed statistically significant benefits of fosinopril sodium therapy, compared to placebo, in one or more of the following: exercise tolerance (one study), symptoms of dyspnea, orthopnea and paroxysmal nocturnal dyspnea (2 studies), NYHA classification (2 studies), hospitalization for heart failure (2 studies), study withdrawals for worsening heart failure (2 studies), and/or need for supplemental diuretics (2 studies). Favorable effects were maintained for up to two years. Effects of fosinopril sodium on long-term mortality in heart failure have not been evaluated. The once daily dosage for the treatment of congestive heart failure was the only dosage regimen used during clinical trial development and was determined by the measurement of hemodynamic responses.
-
INDICATIONS AND USAGEFosinopril sodium tablets are indicated for the treatment of hypertension. It may be used alone or in combination with thiazide diuretics. Fosinopril sodium tablets are indicated in the ...
Fosinopril sodium tablets are indicated for the treatment of hypertension. It may be used alone or in combination with thiazide diuretics.
Fosinopril sodium tablets are indicated in the management of heart failure as adjunctive therapy when added to conventional therapy including diuretics with or without digitalis (see DOSAGE AND ADMINISTRATION).
In using fosinopril sodium, consideration should be given to the fact that another angiotensin converting enzyme inhibitor, captopril, has caused agranulocytosis, particularly in patients with renal impairment or collagen-vascular disease. Available data are insufficient to show that fosinopril sodium does not have a similar risk (see WARNINGS).
In considering use of fosinopril sodium, it should be noted that in controlled trials ACE inhibitors have an effect on blood pressure that is less in black patients than in non-blacks. In addition, ACE inhibitors (for which adequate data are available) cause a higher rate of angioedema in black than in non-black patients (see WARNINGS: Head and Neck Angioedema and Intestinal Angioedema).
Close -
CONTRAINDICATIONSFosinopril sodium tablets are contraindicated in patients who are hypersensitive to this product or to any other angiotensin converting enzyme inhibitor (e.g., a patient who has experienced ...
Fosinopril sodium tablets are contraindicated in patients who are hypersensitive to this product or to any other angiotensin converting enzyme inhibitor (e.g., a patient who has experienced angioedema with any other ACE inhibitor therapy).
Do not co-administer fosinopril sodium tablets with aliskiren in patients with diabetes.
Close -
WARNINGSAnaphylactoid and Possibly Related Reactions - Presumably because angiotensin-converting enzyme inhibitors affect the metabolism of eicosanoids and polypeptides, including endogenous bradykinin ...
Anaphylactoid and Possibly Related Reactions
Presumably because angiotensin-converting enzyme inhibitors affect the metabolism of eicosanoids and polypeptides, including endogenous bradykinin, patients receiving ACE inhibitors (including fosinopril sodium) may be subject to a variety of adverse reactions, some of them serious.
Head and Neck Angioedema: Angioedema involving the extremities, face, lips, mucous membranes, tongue, glottis or larynx has been reported in patients treated with ACE inhibitors. If angioedema involves the tongue, glottis or larynx, airway obstruction may occur and be fatal. If laryngeal stridor or angioedema of the face, lips, mucous membranes, tongue, glottis or extremities occurs, treatment with fosinopril sodium should be discontinued and appropriate therapy instituted immediately. Where there is involvement of the tongue, glottis, or larynx, likely to cause airway obstruction, appropriate therapy, e.g., subcutaneous epinephrine solution 1:1000 (0.3 mL to 0.5 mL) should be promptly administered (see PRECAUTIONS: Information for Patients and ADVERSE REACTIONS). Patients taking concomitant mTOR inhibitor (e.g. temsirolimus) therapy may be at increased risk for angioedema.
Intestinal Angioedema: Intestinal angioedema has been reported in patients treated with ACE inhibitors. These patients presented with abdominal pain (with or without nausea or vomiting); in some cases there was no prior history of facial angioedema and C-1 esterase levels were normal. The angioedema was diagnosed by procedures including abdominal CT scan or ultrasound, or at surgery, and symptoms resolved after stopping the ACE inhibitor. Intestinal angioedema should be included in the differential diagnosis of patients on ACE inhibitors presenting with abdominal pain.
Anaphylactoid reactions during desensitization: Two patients undergoing desensitizing treatment with hymenoptera venom while receiving ACE inhibitors sustained life-threatening anaphylactoid reactions. In the same patients, these reactions were avoided when ACE inhibitors were temporarily withheld, but they reappeared upon inadvertent rechallenge.
Anaphylactoid reactions during membrane exposure: Anaphylactoid reactions have been reported in patients dialyzed with high-flux membranes and treated concomitantly with an ACE inhibitor. Anaphylactoid reactions have also been reported in patients undergoing low-density lipoprotein apheresis with dextran sulfate absorption.
Hypotension
Fosinopril sodium can cause symptomatic hypotension. Like other ACE inhibitors, fosinopril has been only rarely associated with hypotension in uncomplicated hypertensive patients. Symptomatic hypotension is most likely to occur in patients who have been volume-and /or salt-depleted as a result of prolonged diuretic therapy, dietary salt restriction, dialysis, diarrhea, or vomiting. Volume-and/or salt depletion should be corrected before initiating therapy with fosinopril sodium.
In patients with heart failure, with or without associated renal insufficiency, ACE inhibitor therapy may cause excessive hypotension, which may be associated with oliguria or azotemia and, rarely, with acute renal failure and death. In such patients, fosinopril sodium therapy should be started under close medical supervision; they should be followed closely for the first 2 weeks of treatment and whenever the dose of fosinopril or diuretic is increased. Consideration should be given to reducing the diuretic dose in patients with normal or low blood pressure who have been treated vigorously with diuretics or who are hyponatremic.
If hypotension occurs, the patient should be placed in a supine position, and, if necessary, treated with intravenous infusion of physiological saline. Fosinopril sodium treatment usually can be continued following restoration of blood pressure and volume.
Neutropenia/Agranulocytosis
Another angiotensin converting enzyme inhibitor, captopril, has been shown to cause agranulocytosis and bone marrow depression, rarely in uncomplicated patients, but more frequently in patients with renal impairment, especially if they also have a collagen-vascular disease such as systemic lupus erythematosus or scleroderma. Available data from clinical trials of fosinopril are insufficient to show that fosinopril does not cause agranulocytosis at similar rates. Monitoring of white blood cell counts should be considered in patients with collagen-vascular disease, especially if the disease is associated with impaired renal function.
Fetal Toxicity
Use of drugs that act on the renin-angiotensin system during the second and third trimesters of pregnancy reduces fetal renal function and increases fetal and neonatal morbidity and death. Resulting oligohydramnios can be associated with fetal lung hypoplasia and skeletal deformations. Potential neonatal adverse effects include skull hypoplasia, anuria, hypotension, renal failure, and death.
When pregnancy is detected, discontinue fosinopril as soon as possible. These adverse outcomes are usually associated with use of these drugs in the second and third trimester of pregnancy. Most epidemiologic studies examining fetal abnormalities after exposure to antihypertensive use in the first trimester have not distinguished drugs affecting the renin-angiotensin system from other antihypertensive agents. Appropriate management of maternal hypertension during pregnancy is important to optimize outcomes for both mother and fetus.
In the unusual case that there is no appropriate alternative to therapy with drugs affecting the renin-angiotensin system for a particular patient, apprise the mother of the potential risk to the fetus. Perform serial ultrasound examinations to assess the intra-amniotic environment. If oligohydramnios is observed, discontinue fosinopril, unless it is considered lifesaving for the mother. Fetal testing may be appropriate, based on the week of pregnancy. Patients and physicians should be aware, however, that oligohydramnios may not appear until after the fetus has sustained irreversible injury. Closely observe infants with histories of in utero exposure to fosinopril for hypotension, oliguria, and hyperkalemia.[see Precautions, Pediatric Use].
No teratogenic effects of fosinopril were seen in studies of pregnant rats and rabbits. On a mg/kg basis, the doses used were up to 180 times (in rats) and one time (in rabbits) the maximum recommended human dose.
When fosinopril was given to pregnant rats at doses about 80 to 250 times (on a mg/kg basis) the maximum recommended human dose, three similar orofacial malformations and one fetus with situs inversus were observed among the offspring.
CloseHepatic Failure
Rarely, ACE Inhibitors have been associated with a syndrome that starts with cholestatic jaundice and progresses to fulminant hepatic necrosis and (sometimes) death. The mechanism of this syndrome is not understood. Patients receiving ACE inhibitors who develop jaundice or marked elevations of hepatic enzymes should discontinue the ACE inhibitor and receive appropriate medical follow-up.
-
PRECAUTIONSGeneral - Impaired Renal Function: As a consequence of inhibiting the renin-angiotensin-aldosterone system, changes in renal function may be anticipated in susceptible individuals. In patients ...
General
Impaired Renal Function: As a consequence of inhibiting the renin-angiotensin-aldosterone system, changes in renal function may be anticipated in susceptible individuals. In patients with severe congestive heart failure whose renal function may depend on the activity of the renin-angiotensin-aldosterone system, treatment with angiotensin-converting enzyme inhibitors, including fosinopril sodium, may be associated with oliguria and/or progressive azotemia and (rarely) with acute renal failure and/or death.
In hypertensive patients with renal artery stenosis in a solitary kidney or bilateral renal artery stenosis, increases in blood urea nitrogen and serum creatinine may occur. Experience with another angiotensin converting enzyme inhibitor suggests that these increases are usually reversible upon discontinuation of ACE inhibitor and/or diuretic therapy. In such patients, renal function should be monitored during the first few weeks of therapy. Some hypertensive patients with no apparent pre-existing renal vascular disease have developed increases in blood urea nitrogen and serum creatinine, usually minor and transient, especially when fosinopril sodium has been given concomitantly with a diuretic. This is more likely to occur in patients with pre-existing renal impairment. Dosage reduction of fosinopril sodium and/or discontinuation of the diuretic may be required.
Evaluation of patients with hypertension or heart failure should always include assessment of renal function (see DOSAGE AND ADMINISTRATION).
Impaired renal function decreases total clearance of fosinoprilat and approximately doubles AUC. In general, no adjustment of dosing is needed. However, patients with heart failure and severely reduced renal function may be more sensitive to the hemodynamic effects (e.g., hypotension) of ACE inhibition (see CLINICAL PHARMACOLOGY).
Hyperkalemia: In clinical trials, hyperkalemia (serum potassium greater than 10% above the upper limit of normal) has occurred in approximately 2.6% of hypertensive patients receiving fosinopril sodium. In most cases, these were isolated values which resolved despite continued therapy. In clinical trials, 0.1% of patients (two patients) were discontinued from therapy due to an elevated serum potassium. Risk factors for the development of hyperkalemia include renal insufficiency, diabetes mellitus, and the concomitant use of potassium-sparing diuretics, potassium supplements, and/or potassium-containing salt substitutes, which should be used cautiously, if at all, with fosinopril sodium (see PRECAUTIONS: Drug Interactions).
Cough: Presumably due to the inhibition of the degradation of endogenous bradykinin, persistent nonproductive cough has been reported with all ACE inhibitors, always resolving after discontinuation of therapy. ACE inhibitor-induced cough should be considered in the differential diagnosis of cough.
Impaired Liver Function: Since fosinopril is primarily metabolized by hepatic and gut wall esterases to its active moiety, fosinoprilat, patients with impaired liver function could develop elevated plasma levels of unchanged fosinopril. In a study in patients with alcoholic or biliary cirrhosis, the extent of hydrolysis was unaffected, although the rate was slowed. In these patients, the apparent total body clearance of fosinoprilat was decreased and the plasma AUC approximately doubled.
Surgery/Anesthesia: In patients undergoing surgery or during anesthesia with agents that produce hypotension, fosinopril will block the angiotensin II formation that could otherwise occur secondary to compensatory renin release. Hypotension that occurs as a result of this mechanism can be corrected by volume expansion.
Hemodialysis
Recent clinical observations have shown an association of hypersensitivity-like (anaphylactoid) reactions during hemodialysis with high-flux dialysis membranes (e.g., AN69) in patients receiving ACE inhibitors as medication. In these patients, consideration should be given to using a different type of dialysis membrane or a different class of medication. (See WARNINGS: Anaphylactoid reactions during membrane exposure.)
Information for Patients
Angioedema: Angioedema, including laryngeal edema, can occur with treatment with ACE inhibitors, especially following the first dose. Patients should be advised to immediately report to their physician any signs or symptoms suggesting angioedema (e.g., swelling of face, eyes, lips, tongue, larynx, mucous membranes, and extremities; difficulty in swallowing or breathing; hoarseness) and to discontinue therapy. (See WARNINGS: Head and Neck Angioedema and Intestinal Angioedema and ADVERSE REACTIONS.)
Symptomatic Hypotension: Patients should be cautioned that lightheadedness can occur, especially during the first days of therapy, and it should be reported to a physician. Patients should be told that if syncope occurs, fosinopril sodium should be discontinued until the physician has been consulted.
All patients should be cautioned that inadequate fluid intake or excessive perspiration, diarrhea, or vomiting can lead to an excessive fall in blood pressure, with the same consequences of lightheadedness and possible syncope.
Hyperkalemia: Patients should be told not to use potassium supplements or salt substitutes containing potassium without consulting the physician.
Neutropenia: Patients should be told to promptly report any indication of infection (e.g., sore throat, fever), which could be a sign of neutropenia.
Pregnancy: Female patients of childbearing age should be told about the consequences of exposure to fosinopril during pregnancy. Discuss treatment options with women planning to become pregnant. Patients should be asked to report pregnancies to their physicians as soon as possible.
Drug Interactions
With diuretics: Patients on diuretics, especially those with intravascular volume depletion, may occasionally experience an excessive reduction of blood pressure after initiation of therapy with fosinopril sodium. The possibility of hypotensive effects with fosinopril sodium can be minimized by either discontinuing the diuretic or increasing salt intake prior to initiation of treatment with fosinopril sodium. If this is not possible, the starting dose should be reduced and the patient should be observed closely for several hours following an initial dose and until blood pressure has stabilized. (see DOSAGE AND ADMINISTRATION).
With potassium supplements and potassium-sparing diuretics: Fosinopril sodium can attenuate potassium loss caused by thiazide diuretics. Potassium-sparing diuretics (spironolactone, amiloride, triamterene, and others) or potassium supplements can increase the risk of hyperkalemia. Therefore, if concomitant use of such agents is indicated, they should be given with caution, and the patient's serum potassium should be monitored frequently.
With lithium: Increased serum lithium levels and symptoms of lithium toxicity have been reported in patients receiving ACE inhibitors during therapy with lithium. These drugs should be co administered with caution, and frequent monitoring of serum lithium levels is recommended. If a diuretic is also used, the risk of lithium toxicity may be increased.
With antacids: In a clinical pharmacology study, coadministration of an antacid (aluminum hydroxide, magnesium hydroxide, and simethicone) with fosinopril reduced serum levels and urinary excretion of fosinoprilat as compared with fosinopril administrated alone, suggesting that antacids may impair absorption of fosinopril. Therefore, if concomitant administration of these agents is indicated, dosing should be separated by 2 hours.
Gold: Nitritoid reactions (symptoms include facial flushing, nausea, vomiting, and hypotension) have been reported rarely in patients on therapy with injectable gold (sodium aurothiomalate) and concomitant ACE inhibitor therapy including fosinopril sodium.
Non-steroidal anti-inflammatory agents including selective cyclooxygenase-2 inhibitors (COX-2 inhibitors):In patients who are elderly, volume-depleted (including those on diuretic therapy), or with compromised renal function, co-administration of NSAIDs, including selective COX-2 inhibitors, with ACE inhibitors, including fosinopril, may result in deterioration of renal function, including possible acute renal failure. These effects are usually reversible. Monitor renal function periodically in patients receiving quinapril and NSAID therapy.
The antihypertensive effect of ACE inhibitors, including fosinopril may be attenuated by NSAIDs.
Agents that inhibit mTOR: Patients taking concomitant mTOR inhibitor (e.g. temsirolimus) therapy may be at increased risk for angioedema.
Dual Blockade of the Renin-Angiotensin System (RAS):Dual blockade of the RAS with angiotensin receptor blockers, ACE inhibitors, or aliskiren is associated with increased risks of hypotension, hyperkalemia, and changes in renal function (including acute renal failure) compared to monotherapy. Closely monitor blood pressure, renal function and electrolytes in patients on fosinopril and other agents that affect the RAS.
Do not co-administer aliskiren with fosinopril in patients with diabetes. Avoid use of aliskiren with fosinopril in patients with renal impairment (GFR <60 ml/min).
Other: Neither fosinopril sodium nor its metabolites have been found to interact with food. In separate single or multiple dose pharmacokinetic interaction studies with chlorthalidone, nifedipine, propranolol, hydrochlorothiazide, cimetidine, metoclopramide, propantheline, digoxin, and warfarin, the bioavailability of fosinoprilat was not altered by coadministration of fosinopril with any one of these drugs. In a study with concomitant administration of aspirin and fosinopril sodium, the bioavailability of unbound fosinoprilat was not altered.
In a pharmacokinetic interaction study with warfarin, bioavailability parameters, the degree of protein binding, and the anticoagulant effect (measured by prothrombin time) of warfarin were not significantly changed.
Drug/Laboratory Test Interaction
Fosinopril may cause a false low measurement of serum digoxin levels with the Digi-Tab® RIA Kit, for Digoxin. Other Kits, such as the Coat-A-Count® RIA Kit, may be used.
Carcinogenesis, Mutagenesis, and Impairment of Fertility
No evidence of a carcinogenic effect was found when fosinopril was given in the diet to mice and rats for up to 24 months at doses up to 400 mg/kg/day. On a body weight basis, the highest dose in mice and rats is about 250 times the maximum human dose of 80 mg, assuming a 50 kg subject. On a body surface area basis, in mice, this dose is 20 times the maximum human dose; in rats, this dose is 40 times the maximum human dose. Male rats given the highest dose level had a slightly higher incidence of mesentery/omentum lipomas.
Neither fosinopril nor the active fosinoprilat was mutagenic in the Ames microbial mutagen test, the mouse lymphoma forward mutation assay, or a mitotic gene conversion assay. Fosinopril was also not genotoxic in a mouse micronucleus test in vivo and a mouse bone marrow cytogenetic assay in vivo.
In the Chinese hamster ovary cell cytogenetic assay, fosinopril increased the frequency of chromosomal aberrations when tested without metabolic activation at a concentration that was toxic to the cells. However, there was no increase in chromosomal aberrations at lower drug concentrations without metabolic activation or at any concentration with metabolic activation.
There were no adverse reproductive effects in male and female rats treated with 15 or 60 mg/kg daily. On a body weight basis, the high dose of 60 mg/kg is about 38 times the maximum recommended human dose. On a body surface area basis, this dose is 6 times the maximum recommended human dose. There was no effect on pairing time prior to mating in rats until a daily dose of 240 mg/kg, a toxic dose, was given; at this dose, a slight increase in pairing time was observed. On a body weight basis, this dose is 150 times the maximum recommended human dose. On a body surface area basis, this dose is 24 times the maximum recommended human dose.
Nursing Mothers
Ingestion of 20 mg daily for three days resulted in detectable levels of fosinoprilat in breast milk.
Fosinopril sodium should not be administered to nursing mothers.
Geriatric Use
Clinical studies of fosinopril sodium did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
ClosePediatric Use
Neonates with a history of in utero exposure to fosinopril sodium tablets:
If oliguria or hypotension occurs, direct attention toward support of blood pressure and renal perfusion. Exchange transfusions or dialysis may be required as a means of reversing hypotension and/or substituting for disordered renal function. Removal of fosinopril, which crosses the placenta, from the neonatal circulation is not significantly accelerated by these means
The antihypertensive effects of fosinopril have been evaluated in a double-blind study in pediatric patients 6 to 16 years of age (see CLINICAL PHARMACOLOGY: Pharmacodynamics and Clinical Effects, Hypertension). The pharmacokinetics of fosinopril have been evaluated in pediatric patients 6 to 16 years of age (see CLINICAL PHARMACOLOGY: Pharmacokinetics and Metabolism). Fosinopril was generally well tolerated and adverse effects were similar to those described in adults (see ADVERSE REACTIONS: Pediatric Patients).
-
ADVERSE REACTIONSFosinopril sodium has been evaluated for safety in more than 2100 individuals in hypertension and heart failure trials, including approximately 530 patients treated for a year or more. Generally ...
Fosinopril sodium has been evaluated for safety in more than 2100 individuals in hypertension and heart failure trials, including approximately 530 patients treated for a year or more. Generally adverse events were mild and transient, and their frequency was not prominently related to dose within the recommended daily dosage range.
Hypertension
In placebo-controlled clinical trials (688 fosinopril sodium -treated patients), the usual duration of therapy was two to three months. Discontinuations due to any clinical or laboratory adverse event were 4.1% and 1.1% in fosinopril sodium-treated and placebo-treated patients, respectively. The most frequent reasons (0.4 to 0.9%) were headache, elevated transaminases, fatigue, cough (see PRECAUTIONS: General, Cough), diarrhea, and nausea and vomiting
During clinical trials with any fosinopril sodium regimen, the incidence of adverse events in the elderly (≥65 years old) was similar to that seen in younger patients
Clinical adverse events probably or possibly related or of uncertain relationship to therapy, occurring in at least 1% of patients treated with fosinopril sodium alone and at least as frequent on fosinopril sodium as on placebo in placebo-controlled clinical trials are shown in the table below.
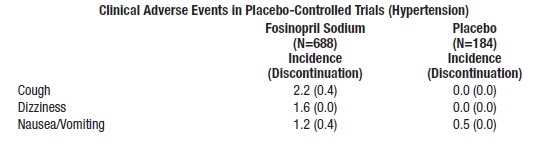
The following events were also seen at >1% on fosinopril sodium but occurred in the placebo group at a greater rate: headache, diarrhea, fatigue, and sexual dysfunction. Other clinical events probably or possibly related, or of uncertain relationship to therapy occurring in 0.2 to 1.0% of patients (excepte as noted) treated with fosinopril sodium in controlled or uncontrolled clinical trials (N=1479) and less frequent, clinically significant events include (listed by body system):
General: Chest pain, edema, weakness, excessive sweating.
Cardiovascular: Angina/myocardial infarction, cerebrovascular accident, hypertensive crisis, rhythm disturbances, palpitations, hypotension, syncope, flushing, claudication.
Orthostatic hypotension occurred in 1.4% of patients treated with fosinopril monotherapy. Hypotension or orthostatic hypotension was a cause for discontinuation of therapy in 0.1% of patients.
Dermatologic: Urticaria, rash, photosensitivity, pruritus.
Endocrine/Metabolic: Gout, decreased libido.
Gastrointestinal: Pancreatitis, hepatitis, dysphagia, abdominal distention, abdominal pain, flatulence, constipation, heartburn, appetite/weight change, dry mouth.
Hematologic: Lymphadenopathy.
Immunologic: Angioedema. (See WARNINGS: Head and Neck Angioedema and Intestinal Angioedema.)
Musculoskeletal: Arthralgia, musculoskeletal pain, myalgia/muscle cramp.
Nervous/Psychiatric: Memory disturbance, tremor, confusion, mood change, paresthesia, sleep disturbance, drowsiness, vertigo.
Respiratory: Bronchospasm, pharyngitis, sinusitis/rhinitis, laryngitis/hoarseness, epistaxis. A symptom-complex of cough, bronchospasm, and eosinophilia has been observed in two patients treated with fosinopril.
Special Senses: Tinnitus, vision disturbance, taste disturbance, eye irritation.
Urogenital: Renal insufficiency, urinary frequency.
Heart Failure
In placebo-controlled clinical trials (361 fosinopril sodium -treated patients), the usual duration of therapy was 3 to 6 months. Discontinuations due to any clinical or laboratory adverse event, except for heart failure, were 8.0% and 7.5% in fosinopril sodium-treated and placebo-treated patients, respectively. The most frequent reason for discontinuation of fosinopril sodium was angina pectoris (1.1 %). Significant hypotension after the first dose of fosinopril sodium occurred in 14/590 (2.4%) of patients; 5/590 (0.8%) patients discontinued due to first dose hypotension.
Clinical adverse events probably or possibly related or of uncertain relationship to therapy, occurring in at least 1% of patients treated with fosinopril sodium and at least as common as the placebo group, in placebo-controlled trials are shown in the table below.
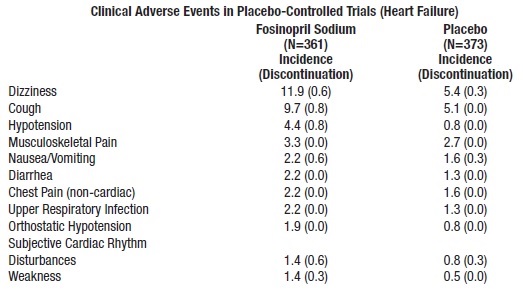
The following events also occurred at a rate of 1% or more on fosinopril sodium, but occurred on placebo more often: fatigue, dyspnea, headache, rash, abdominal pain, muscle cramp, angina pectoris, edema, and insomnia.
The incidence of adverse events in the elderly (≥65 years old) was similar to that seen in younger patients.
Other clinical events probably or possibly related, or of uncertain relationship to therapy occurring in 0.4 to 1.0% of patients (except as noted) treated with fosinopril sodium in controlled clinical trials (N=516) and less frequent, clinically significant events include (listed by body system):
General: Fever, influenza, weight gain, hyperhidrosis, sensation of cold, fall, pain.
Cardiovascular: Sudden death cardiorespiratory arrest, shock (0.2%), atrial rhythm disturbance, cardiac rhythm disturbances, non anginal chest pain, edema lower extremity, hypertension, syncope, conduction disorder, bradycardia, tachycardia.
Dermatologic: Urticaria, rash, photosensitivity, pruritus.
Endocrine/Metabolic: Gout, sexual dysfunction.
Gastrointestinal: Hepatomegaly, abdominal distension, decreased appetite, dry mouth, constipation, flatulence.
Immunologic: Angioedema (0.2%).
Musculoskeletal: Muscle ache, swelling of an extremity, weakness of an extremity.
Nervous/Psychiatric: Cerebral infarction, TIA, depression, numbness, paresthesia, vertigo, behavior change, tremor.
Respiratory: Abnormal vocalization, rhinitis, sinus abnormality, tracheobronchitis, abnormal breathing, pleuritic chest pain.
Special Senses: Vision disturbance, taste disturbance.
Urogenital: Abnormal urination, kidney pain.
Potential Adverse Effects Reported with ACE Inhibitors
Body as a whole: Anaphylactoid reactions (see WARNINGS: Anaphylactoid and possible related reactions and PRECAUTIONS: Hemodialysis).
Other medically important adverse effects reported with ACE inhibitors include: Cardiac arrest; eosinophilic pneumonitis; neutropenia/agranulocytosis, pancytopenia, anemia (including hemolytic and aplastic), thrombocytopenia; acute renal failure; hepatic failure, jaundice (hepatocellular or cholestatic); symptomatic hyponatremia; bullous pemphigus, exfoliative dermatitis; a syndrome which may include: arthralgia/arthritis, vasculitis, serositis, myalgia, fever, rash or other dermatologic manifestations, a positive ANA, leukocytosis, eosinophilia, or an elevated ESR.
Laboratory Test Abnormalities
Serum Electrolytes: Hyperkalemia, (see PRECAUTIONS); hyponatremia, (see PRECAUTIONS: Drug Interactions; With diuretics).
BUN/Serum Creatinine: Elevations, usually transient and minor, of BUN or serum creatinine have been observed. In placebo-controlled clinical trials, there were no significant differences in the number of patients experiencing increases in serum creatinine (outside the normal range or 1.33 times the pre-treatment value) between the fosinopril and placebo treatment groups. Rapid reduction of longstanding or markedly elevated blood pressure by any antihypertensive therapy can result in decreases in the glomerular filtration rate and, in turn, lead to increases in BUN or serum creatinine. (See PRECAUTIONS: General.)
Hematology: In controlled trials, a mean hemoglobin decrease of 0.1 g/dL was observed in fosinopril treated patients. In individual patients decreases in hemoglobin or hematocrit were usually transient, small, and not associated with symptoms. No patient was discontinued from therapy due to the development of anemia. Other: Neutropenia (see WARNINGS), leukopenia and eosinophilia.
Liver Function Tests: Elevations of transaminases, LDH, alkaline phosphatase and serum bilirubin have been reported. Fosinopril therapy was discontinued because of serum transaminase elevations in 0.7% of patients. In the majority of cases, the abnormalities were either present at baseline or were associated with other etiologic factors. In those cases which were possibly related to fosinopril therapy, the elevations were generally mild and transient and resolved after discontinuation of therapy.
ClosePediatric Patients
The adverse experience profile for pediatric patients is similar to that seen in adult patients with hypertension. The long-term effects of fosinopril sodium on growth and development have not been studied.
-
OVERDOSAGEOral doses of fosinopril at 2600 mg/kg in rats were associated with significant lethality. Human overdoses of fosinopril have not been reported, but the most common manifestation of human ...
Oral doses of fosinopril at 2600 mg/kg in rats were associated with significant lethality. Human overdoses of fosinopril have not been reported, but the most common manifestation of human fosinopril over dosage is likely to be hypotension.
Laboratory determinations of serum levels of fosinoprilat and its metabolites are not widely available, and such determinations have, in any event, no established role in the management of fosinopril overdose. No data are available to suggest physiological maneuvers (e.g., maneuvers to change the pH of the urine) that might accelerate elimination of fosinopril and its metabolites. Fosinoprilat is poorly removed from the body by both hemodialysis and peritoneal dialysis.
Angiotensin II could presumably serve as a specific antagonist-antidote in the setting of fosinopril overdose, but angiotensin II is essentially unavailable outside of scattered research facilities. Because the hypotensive effect of fosinopril is achieved through vasodilation and effective hypovolemia, it is reasonable to treat fosinopril overdose by infusion of normal saline solution.
No adverse clinical events were reported in 23 pediatric patients, age 6 months to 6 years, given a single 0.3 mg/kg oral dose of fosinopril.
There is a published report of a 20 month-old female, weighing 12 kg, who ingested approximately 200 mg fosinopril sodium. After receiving gastric lavage and activated charcoal within one hour of the ingestion, she made an uneventful recovery.
Close -
DOSAGE AND ADMINISTRATIONHypertension - Adults - The recommended initial dose of fosinopril sodium tablets is 10 mg once a day, both as monotherapy and when the drug is added to a diuretic. Dosage should then be adjusted ...
Adults
The recommended initial dose of fosinopril sodium tablets is 10 mg once a day, both as monotherapy and when the drug is added to a diuretic. Dosage should then be adjusted according to blood pressure response at peak (2 to 6 hours) and trough (about 24 hours after dosing) blood levels. The usual dosage range needed to maintain a response at trough is 20 mg to 40 mg but some patients appear to have a further response to 80 mg. In some patients treated with once daily dosing, the antihypertensive effect may diminish toward the end of the dosing interval. If trough response is inadequate, dividing the daily dose should be considered. If blood pressure is not adequately controlled with fosinopril sodium alone, a diuretic may be added.
Concomitant administration of fosinopril sodium with potassium supplements, potassium salt substitutes, or potassium-sparing diuretics can lead to increases of serum potassium (see PRECAUTIONS).
In patients who are currently being treated with a diuretic, symptomatic hypotension occasionally can occur following the initial dose of fosinopril sodium tablets. To reduce the likelihood of hypotension, the diuretic should, if possible, be discontinued two to three days prior to beginning therapy with fosinopril sodium tablets (see WARNINGS). Then, if blood pressure is not controlled with fosinopril sodium tablets alone diuretic therapy should be resumed. If diuretic therapy cannot be discontinued, an initial dose of 10 mg of fosinopril sodium tablets should be used with careful medical supervision for several hours and until blood pressure has stabilized. (See WARNINGS and PRECAUTIONS: Information for Patients and Drug Interactions).
Since concomitant administration of fosinopril sodium tablets with potassium supplements, or potassium containing salt substitutes or potassium-sparing diuretics may lead to increases in serum potassium, they should be used with caution (see PRECAUTIONS).
Pediatrics
In children, doses of fosinopril sodium tablets between 0.1 and 0.6 mg/kg have been studied and shown to reduce blood pressure to a similar extent (see CLINICAL PHARMACOLOGY Pharmacodynamics and Clinical Effects ). Based on this, the recommended dose of fosinopril sodium tablets USP in children weighing more than 50 kg is 5 to 10 mg once per day as monotherapy. An appropriate dosage strength is not available for children weighing less than 50 kg.
Heart Failure
Digitalis is not required for fosinopril sodium tablets to manifest improvements in exercise tolerance and symptoms. Most placebo-controlled clinical trial experience has been with both digitalis and diuretics present as background therapy.
The usual starting dose of fosinopril sodium tablets should be 10 mg once daily. Following the initial dose of fosinopril sodium tablets, the patient should be observed under medical supervision for at least two hours for the presence of hypotension or orthostasis and, if present, until blood pressure stabilizes. An initial dose of 5 mg is preferred in heart failure patients with moderate to severe renal failure or those who have been vigorously diuresed.
Dosage should be increased, over a several week period, to a dose that is maximal and tolerated but not exceeding 40 mg once daily. The usual effective dosage range is 20 mg to 40 mg once daily.
The appearance of hypotension, orthostasis, or azotemia early in dose titration should not preclude further careful dose titration. Consideration should be given to reducing the dose of concomitant diuretic.
For Hypertensive or Heart Failure Patients With Renal Impairment: In patients with impaired renal function, the total body clearance of fosinoprilat is approximately 50% slower than in patients with normal renal function. Since hepatobiliary elimination partially compensates for diminished renal elimination, the total body clearance of fosinoprilat does not differ appreciably with any degree of renal insufficiency (creatinine clearances <80 mL/min/1.73m2), including end-stage renal failure (creatinine clearance <10 mL/min/1.73m2) This relative constancy of body clearance of active fosinoprilat, resulting from the dual route of elimination, permits use of the usual dose in patients with any degree of renal impairment. (See WARNINGS: Anaphylactoid reactions during membrane exposure and PRECAUTIONS: Hemodialysis.)
Close -
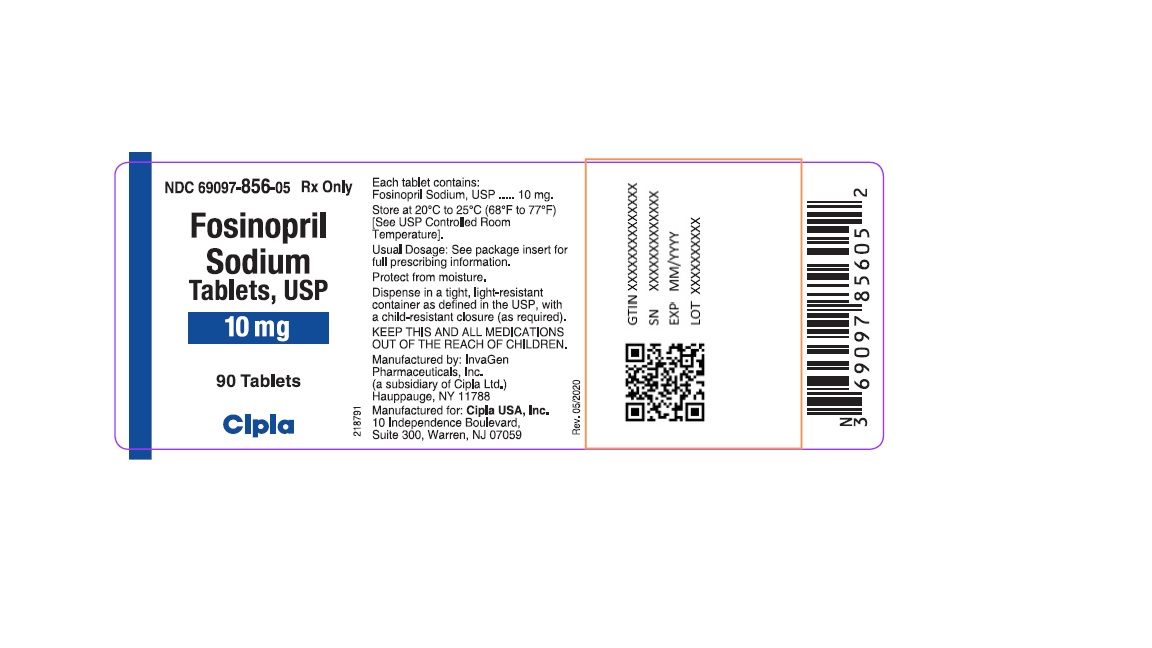
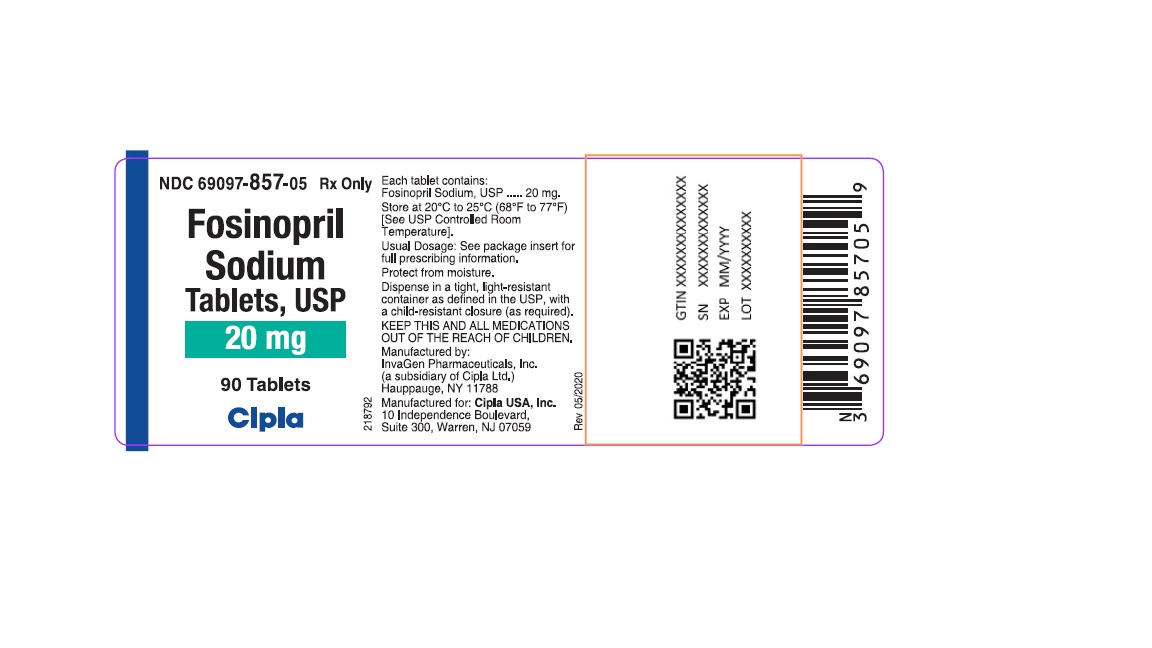
HOW SUPPLIEDFosinopril Sodium Tablets, USP - 10 mg tablets: White, round, biconvex partially scored tablets debossed with "IG" on one side and "200" on other side. They are supplied in bottles of 90 (NDC ...
Fosinopril Sodium Tablets, USP
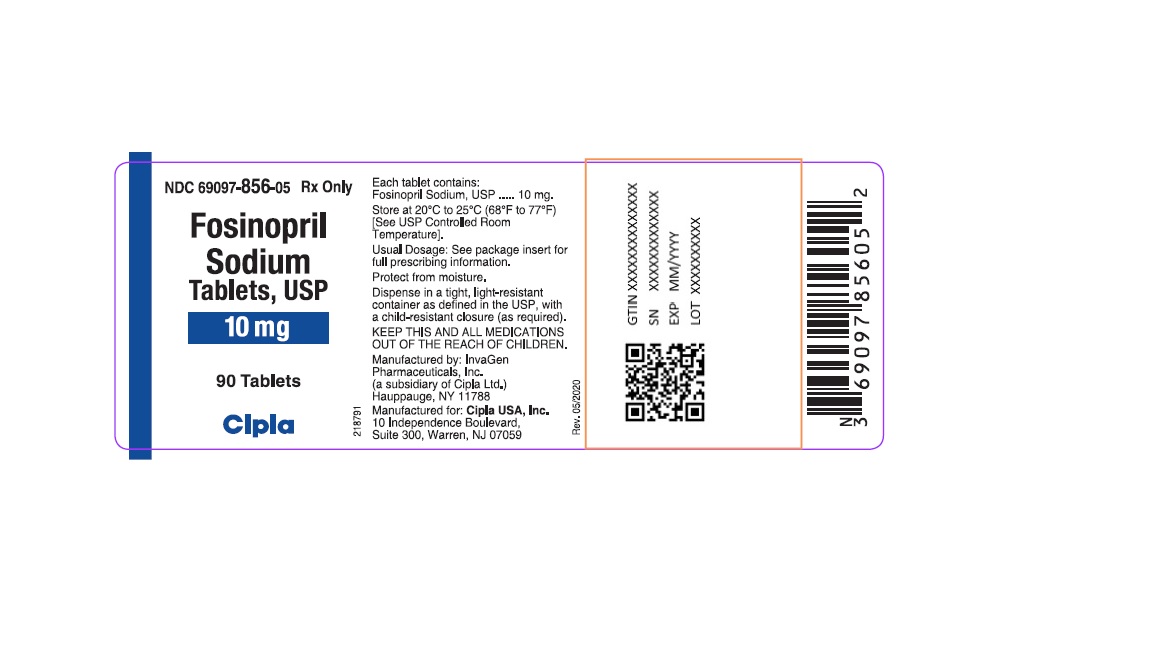
10 mg tablets: White, round, biconvex partially scored tablets debossed with "IG" on one side and "200" on other side. They are supplied in bottles of 90 (NDC 69097-856-05) and 1000 (NDC 69097-856-15). Bottles contain a desiccant canister.
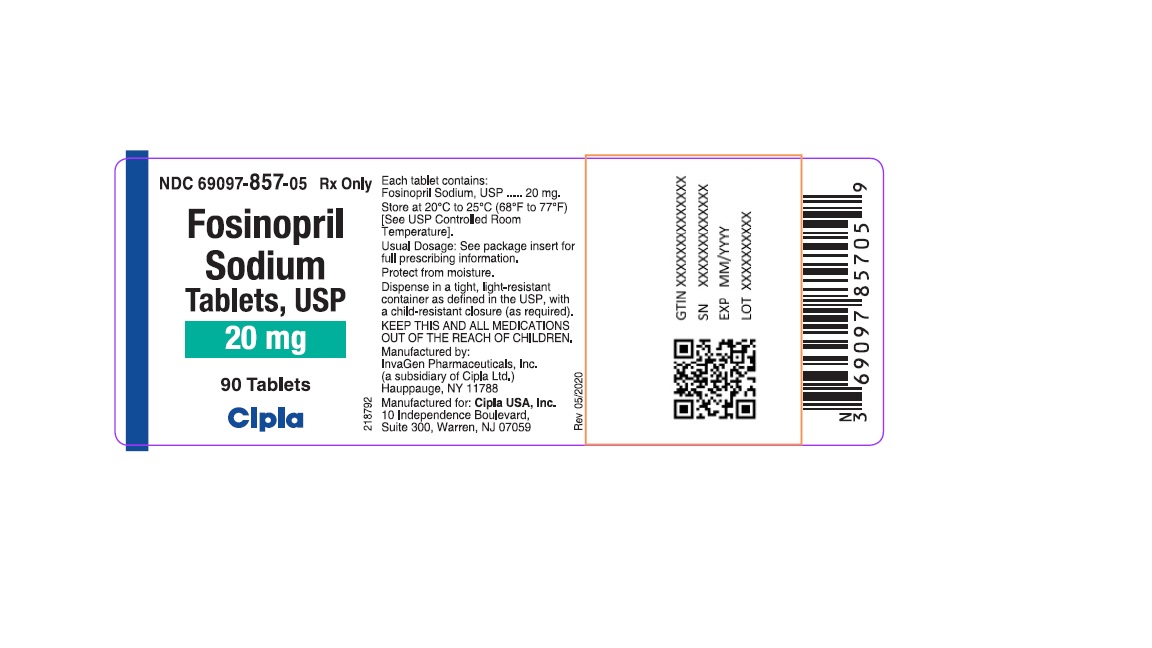
20 mg tablets: White, round, biconvex tablets debossed with "IG" on one side and "201" on other side. They are supplied in bottles of 90 (NDC 69097-857-05) and 1000 (NDC 69097-857-15). Bottles contain a desiccant canister.
40 mg tablets: White, round, biconvex tablets debossed with "IG" on one side and "202" on other side. They are supplied in bottles of 90 (NDC 69097-858-05) and 1000 (NDC 69097-858-15). Bottles contain a desiccant canister.
STORAGE
Store at 20° C to 25° C (68° to 77°F) [see USP Controlled Room Temperature]. Protect from moisture by keeping bottle tightly closed.
Dispense in a tight, light-resistant container as defined in the USP, with a child-resistant closure (as required).Manufactured by:
InvaGen Pharmaceuticals, Inc.
(a subsidiary of Cipla Ltd.)
Hauppauge, NY 11788
Manufactured for:
Cipla USA, Inc.
10 Independence Boulevard, Suite 300
Warren, NJ 07059
Close
Revised: 05/2020 -
PACKAGE LABEL.PRINCIPAL DISPLAY PANELNDC 69097-856-05 Rx Only - Fosinopril - Sodium - Tablets, USP - 10 mg - 90 Tablets - Cipla - NDC 69097-857-05 Rx ...
Fosinopril
Sodium
Tablets, USP
10 mg
90 Tablets
Cipla
Fosinopril
Sodium
Tablets, USP
20 mg
90 Tablets
Cipla
Fosinopril
Sodium
Tablets, USP
40 mg
90 Tablets
Cipla
Close
-
INGREDIENTS AND APPEARANCEProduct Information
FOSINOPRIL SODIUM fosinopril tablet Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:69097-856 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FOSINOPRIL SODIUM (UNII: NW2RTH6T2N) (FOSINOPRILAT - UNII:S312EY6ZT8) FOSINOPRIL SODIUM 10 mg Inactive Ingredients Ingredient Name Strength CROSPOVIDONE, UNSPECIFIED (UNII: 2S7830E561) LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) MAGNESIUM STEARATE (UNII: 70097M6I30) POVIDONE, UNSPECIFIED (UNII: FZ989GH94E) Product Characteristics Color WHITE Score 2 pieces Shape ROUND Size 6mm Flavor Imprint Code IG;200 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69097-856-05 90 in 1 BOTTLE; Type 0: Not a Combination Product 06/16/2016 2 NDC:69097-856-15 1000 in 1 BOTTLE; Type 0: Not a Combination Product 06/16/2016 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA077222 06/16/2016 FOSINOPRIL SODIUM fosinopril tablet Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:69097-857 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FOSINOPRIL SODIUM (UNII: NW2RTH6T2N) (FOSINOPRILAT - UNII:S312EY6ZT8) FOSINOPRIL SODIUM 20 mg Inactive Ingredients Ingredient Name Strength CROSPOVIDONE, UNSPECIFIED (UNII: 2S7830E561) LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) MAGNESIUM STEARATE (UNII: 70097M6I30) POVIDONE, UNSPECIFIED (UNII: FZ989GH94E) Product Characteristics Color WHITE Score no score Shape ROUND Size 7mm Flavor Imprint Code IG;201 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69097-857-05 90 in 1 BOTTLE; Type 0: Not a Combination Product 06/16/2016 2 NDC:69097-857-15 1000 in 1 BOTTLE; Type 0: Not a Combination Product 06/16/2016 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA077222 06/16/2016 FOSINOPRIL SODIUM fosinopril tablet Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:69097-858 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FOSINOPRIL SODIUM (UNII: NW2RTH6T2N) (FOSINOPRILAT - UNII:S312EY6ZT8) FOSINOPRIL SODIUM 40 mg Inactive Ingredients Ingredient Name Strength CROSPOVIDONE, UNSPECIFIED (UNII: 2S7830E561) LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) MAGNESIUM STEARATE (UNII: 70097M6I30) POVIDONE, UNSPECIFIED (UNII: FZ989GH94E) Product Characteristics Color WHITE Score no score Shape ROUND Size 10mm Flavor Imprint Code IG;202 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69097-858-05 90 in 1 BOTTLE; Type 0: Not a Combination Product 06/16/2016 2 NDC:69097-858-15 1000 in 1 BOTTLE; Type 0: Not a Combination Product 06/16/2016 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA077222 06/16/2016 Labeler - Cipla USA Inc. (078719707) Registrant - Cipla USA Inc. (078719707) Establishment Name Address ID/FEI Business Operations InvaGen Pharmaceutical Inc 165104469 analysis(69097-856, 69097-857, 69097-858) , manufacture(69097-856, 69097-857, 69097-858)
CloseEstablishment Name Address ID/FEI Business Operations InvaGen Pharmaceuticals, Inc 080334903 pack(69097-856, 69097-857, 69097-858) , analysis(69097-856, 69097-857, 69097-858)
Find additional resources
(also available in the left menu)Safety
Boxed Warnings, Report Adverse Events, FDA Safety Recalls, Presence in Breast Milk
Related Resources
Medline Plus, Clinical Trials, PubMed, Biochemical Data Summary
More Info on this Drug
View Labeling Archives, RxNorm, Get Label RSS Feed, View NDC Code(s)NEW!
RxNorm
FOSINOPRIL SODIUM- fosinopril tablet
| RxCUI | RxNorm NAME | RxTTY | |
|---|---|---|---|
| 1 | 857169 | fosinopril sodium 10 MG Oral Tablet | PSN |
| 2 | 857169 | fosinopril sodium 10 MG Oral Tablet | SCD |
| 3 | 857169 | FNP Sodium 10 MG Oral Tablet | SY |
| 4 | 857183 | fosinopril sodium 20 MG Oral Tablet | PSN |
| 5 | 857183 | fosinopril sodium 20 MG Oral Tablet | SCD |
| 6 | 857183 | FNP Sodium 20 MG Oral Tablet | SY |
| 7 | 857187 | fosinopril sodium 40 MG Oral Tablet | PSN |
| 8 | 857187 | fosinopril sodium 40 MG Oral Tablet | SCD |
| 9 | 857187 | FNP Sodium 40 MG Oral Tablet | SY |
Get Label RSS Feed for this Drug
FOSINOPRIL SODIUM- fosinopril tablet
To receive this label RSS feed
Copy the URL below and paste it into your RSS Reader application.
https://dailymed.nlm.nih.gov/dailymed/labelrss.cfm?setid=736fe52f-69fb-48b2-b1c5-f06531878538
To receive all DailyMed Updates for the last seven days
Copy the URL below and paste it into your RSS Reader application.
https://dailymed.nlm.nih.gov/dailymed/rss.cfm
What will I get with the DailyMed RSS feed?
DailyMed will deliver notification of updates and additions to Drug Label information currently shown on this site through its RSS feed.
DailyMed will deliver this notification to your desktop, Web browser, or e-mail depending on the RSS Reader you select to use. To view updated drug label links, paste the RSS feed address (URL) shown below into a RSS reader, or use a browser which supports RSS feeds, such as Safari for Mac OS X.
How to discontinue the RSS feed
If you no longer wish to have this DailyMed RSS service, simply delete the copied URL from your RSS Reader.
More about getting RSS News & Updates from DailyMedWhy is DailyMed no longer displaying pill images on the Search Results and Drug Info pages?
Due to inconsistencies between the drug labels on DailyMed and the pill images provided by RxImage, we no longer display the RxImage pill images associated with drug labels.
We anticipate reposting the images once we are able identify and filter out images that do not match the information provided in the drug labels.
NDC Codes
FOSINOPRIL SODIUM- fosinopril tablet
If this SPL contains inactivated NDCs listed by the FDA initiated compliance action, they will be specified as such.
| NDC | |
|---|---|
| 1 | 69097-856-05 |
| 2 | 69097-856-15 |
| 3 | 69097-857-05 |
| 4 | 69097-857-15 |
| 5 | 69097-858-05 |
| 6 | 69097-858-15 |