Label: QUINAPRIL tablet
-
NDC Code(s):
69097-839-05,
69097-839-15,
69097-841-05,
69097-841-15, view more69097-842-05, 69097-842-15, 69097-843-05, 69097-843-15
- Packager: Cipla USA Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated January 19, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
BOXED WARNING
(What is this?)
- When pregnancy is detected, discontinue quinapril as soon as possible.
- Drugs that act directly on the renin-angiotensin system can cause injury and death to the developing fetus. See Warnings: Fetal Toxicity
-
DESCRIPTIONQuinapril hydrochloride, USP is the hydrochloride salt of quinapril, the ethyl ester of a non-sulfhydryl, angiotensin-converting enzyme (ACE) inhibitor, quinaprilat. Quinapril hydrochloride ...Close
Quinapril hydrochloride, USP is the hydrochloride salt of quinapril, the ethyl ester of a non-sulfhydryl, angiotensin-converting enzyme (ACE) inhibitor, quinaprilat.
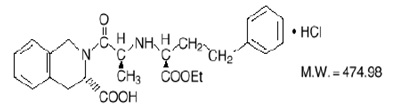
Quinapril hydrochloride, USP is chemically described as [3S-[2[R*(R*)], 3R*]]-2-[2-[1-(ethoxycarbonyl)-3-phenylpropyl]amino]-1-oxopropyl]-1,2,3,4-tetrahydro-3-isoquinolinecarboxylic acid, monohydrochloride. Its empirical formula is C25H30N2O5 •HCl and its structural formula is:
Quinapril hydrochloride, USP is a white to off-white amorphous powder that is freely soluble in aqueous solvents.
Quinapril tablets, USP contain quinapril hydrochloride equivalent to 5 mg, 10 mg, 20 mg, or 40 mg of quinapril for oral administration. Each tablet also contains lactose monohydrate, magnesium carbonate, magnesium stearate, crospovidone, povidone and opadry brown (hypromellose, titanium dioxide, iron oxide and macrogol).
-
CLINICAL PHARMACOLOGYMechanism of Action: Quinapril is deesterified to the principal metabolite, quinaprilat, which is an inhibitor of ACE activity in human subjects and animals. ACE is a peptidyl dipeptidase that ...Close
Mechanism of Action: Quinapril is deesterified to the principal metabolite, quinaprilat, which is an inhibitor of ACE activity in human subjects and animals. ACE is a peptidyl dipeptidase that catalyzes the conversion of angiotensin I to the vasoconstrictor, angiotensin II. The effect of quinapril in hypertension and in congestive heart failure (CHF) appears to result primarily from the inhibition of circulating and tissue ACE activity, thereby reducing angiotensin II formation. Quinapril inhibits the elevation in blood pressure caused by intravenously administered angiotensin I, but has no effect on the pressor response to angiotensin II, norepinephrine or epinephrine. Angiotensin II also stimulates the secretion of aldosterone from the adrenal cortex, thereby facilitating renal sodium and fluid reabsorption. Reduced aldosterone secretion by quinapril may result in a small increase in serum potassium. In controlled hypertension trials, treatment with quinapril alone resulted in mean increases in potassium of 0.07 mmol/L (see PRECAUTIONS). Removal of angiotensin II negative feedback on renin secretion leads to increased plasma renin activity (PRA).
While the principal mechanism of antihypertensive effect is thought to be through the renin- angiotensin-aldosterone system, quinapril exerts antihypertensive actions even in patients with low renin hypertension. Quinapril was an effective antihypertensive in all races studied, although it was somewhat less effective in blacks (usually a predominantly low renin group) than in nonblacks. ACE is identical to kininase II, an enzyme that degrades bradykinin, a potent peptide vasodilator; whether increased levels of bradykinin play a role in the therapeutic effect of quinapril remains to be elucidated.
Pharmacokinetics and Metabolism: Following oral administration, peak plasma quinapril concentrations are observed within one hour. Based on recovery of quinapril and its metabolites in urine, the extent of absorption is at least 60%. The rate and extent of quinapril absorption are diminished moderately (approximately 25 to 30%) when quinapril tablets are administered during a high-fat meal. Following absorption, quinapril is deesterified to its major active metabolite, quinaprilat (about 38% of oral dose), and to other minor inactive metabolites. Following multiple oral dosing of quinapril, there is an effective accumulation half-life of quinaprilat of approximately 3 hours, and peak plasma quinaprilat concentrations are observed approximately 2 hours post-dose. Quinaprilat is eliminated primarily by renal excretion, up to 96% of an IV dose, and has an elimination half-life in plasma of approximately 2 hours and a prolonged terminal phase with a half-life of 25 hours. The pharmacokinetics of quinapril and quinaprilat are linear over a single-dose range of 5 to 80 mg doses and 40 to 160 mg in multiple daily doses. Approximately 97% of either quinapril or quinaprilat circulating in plasma is bound to proteins.
In patients with renal insufficiency, the elimination half-life of quinaprilat increases as creatinine clearance decreases. There is a linear correlation between plasma quinaprilat clearance and creatinine clearance. In patients with end-stage renal disease, chronic hemodialysis or continuous ambulatory peritoneal dialysis has little effect on the elimination of quinapril and quinaprilat. Elimination of quinaprilat may be reduced in elderly patients (≥65 years) and in those with heart failure; this reduction is attributable to decrease in renal function (see DOSAGE AND ADMINISTRATION). Quinaprilat concentrations are reduced in patients with alcoholic cirrhosis due to impaired deesterification of quinapril. Studies in rats indicate that quinapril and its metabolites do not cross the blood-brain barrier.
Pharmacodynamics and Clinical Effects
Hypertension: Single doses of 20 mg of quinapril provide over 80% inhibition of plasma ACE for 24 hours. Inhibition of the pressor response to angiotensin I is shorter-lived, with a 20 mg dose giving 75% inhibition for about 4 hours, 50% inhibition for about 8 hours, and 20% inhibition at 24 hours. With chronic dosing, however, there is substantial inhibition of angiotensin II levels at 24 hours by doses of 20 to 80 mg.
Administration of 10 to 80 mg of quinapril to patients with mild to severe hypertension results in a reduction of sitting and standing blood pressure to about the same extent with minimal effect on heart rate. Symptomatic postural hypotension is infrequent although it can occur in patients who are salt-and/or volume-depleted (see WARNINGS). Antihypertensive activity commences within 1 hour with peak effects usually achieved by 2 to 4 hours after dosing. During chronic therapy, most of the blood pressure lowering effect of a given dose is obtained in 1 to 2 weeks. In multiple-dose studies, 10 to 80 mg per day in single or divided doses lowered systolic and diastolic blood pressure throughout the dosing interval, with a trough effect of about 5 to 11/3 to 7 mm Hg. The trough effect represents about 50% of the peak effect. While the dose-response relationship is relatively flat, doses of 40 to 80 mg were somewhat more effective at trough than 10 to 20 mg, and twice daily dosing tended to give a somewhat lower trough blood pressure than once daily dosing with the same total dose. The antihypertensive effect of quinapril continues during long-term therapy, with no evidence of loss of effectiveness.
Hemodynamic assessments in patients with hypertension indicate that blood pressure reduction produced by quinapril is accompanied by a reduction in total peripheral resistance and renal vascular resistance with little or no change in heart rate, cardiac index, renal blood flow, glomerular filtration rate, or filtration fraction.
Use of quinapril with a thiazide diuretic gives a blood-pressure lowering effect greater than that seen with either agent alone.
In patients with hypertension, quinapril 10 to 40 mg was similar in effectiveness to captopril, enalapril, propranolol, and thiazide diuretics.
Therapeutic effects appear to be the same for elderly (≥65 years of age) and younger adult patients given the same daily dosages, with no increase in adverse events in elderly patients.
Heart Failure: In a placebo-controlled trial involving patients with congestive heart failure treated with digitalis and diuretics, parenteral quinaprilat, the active metabolite of quinapril, reduced pulmonary capillary wedge pressure and systemic vascular resistance and increased cardiac output/index. Similar favorable hemodynamic effects were seen with oral quinapril in baseline-controlled trials, and such effects appeared to be maintained during chronic oral quinapril therapy. Quinapril reduced renal hepatic vascular resistance and increased renal and hepatic blood flow with glomerular filtration rate remaining unchanged.
A significant dose response relationship for improvement in maximal exercise tolerance has been observed with quinapril therapy. Beneficial effects on the severity of heart failure as measured by New York Heart Association (NYHA) classification and Quality of Life and on symptoms of dyspnea, fatigue, and edema were evident after 6 months in a double-blind, placebo-controlled study. Favorable effects were maintained for up to two years of open label therapy. The effects of quinapril on long-term mortality in heart failure have not been evaluated.
-
INDICATIONS AND USAGEHypertension - Quinapril is indicated for the treatment of hypertension, to lower blood pressure. Lowering blood pressure reduces the risk of fatal and nonfatal cardiovascular events ...Close
Quinapril is indicated for the treatment of hypertension, to lower blood pressure. Lowering blood pressure reduces the risk of fatal and nonfatal cardiovascular events, primarily strokes and myocardial infarctions. These benefits have been seen in controlled trials of antihypertensive drugs from a wide variety of pharmacologic classes including the class to which this drug principally belongs. There are no controlled trials demonstrating risk reduction with quinapril.
Control of high blood pressure should be part of comprehensive cardiovascular risk management, including, as appropriate, lipid control, diabetes management, antithrombotic therapy, smoking cessation, exercise, and limited sodium intake. Many patients will require more than one drug to achieve blood pressure goals. For specific advice on goals and management, see published guidelines, such as those of the National High Blood Pressure Education Program's Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC).
Numerous antihypertensive drugs, from a variety of pharmacologic classes and with different mechanisms of action, have been shown in randomized controlled trials to reduce cardiovascular morbidity and mortality, and it can be concluded that it is blood pressure reduction, and not some other pharmacologic property of the drugs, that is largely responsible for those benefits. The largest and most consistent cardiovascular outcome benefit has been a reduction in the risk of stroke, but reductions in myocardial infarction and cardiovascular mortality also have been seen regularly.
Elevated systolic or diastolic pressure causes increased cardiovascular risk, and the absolute risk increase per mmHg is greater at higher blood pressures, so that even modest reductions of severe hypertension can provide substantial benefit. Relative risk reduction from blood pressure reduction is similar across populations with varying absolute risk, so the absolute benefit is greater in patients who are at higher risk independent of their hypertension (for example, patients with diabetes or hyperlipidemia), and such patients would be expected to benefit from more aggressive treatment to a lower blood pressure goal.
Some antihypertensive drugs have smaller blood pressure effects (as monotherapy) in black patients, and many antihypertensive drugs have additional approved indications and effects (e.g., on angina, heart failure, or diabetic kidney disease). These considerations may guide selection of therapy.
Quinapril may be used alone or in combination with thiazide diuretics.
Heart Failure
Quinapril is indicated in the management of heart failure as adjunctive therapy when added to conventional therapy including diuretics and/or digitalis.
In using quinapril, consideration should be given to the fact that another ACE inhibitor, captopril, has caused agranulocytosis, particularly in patients with renal impairment or collagen vascular disease. Available data are insufficient to show that quinapril does not have a similar risk (see WARNINGS).
Angioedema in black patients: Black patients receiving ACE inhibitor monotherapy have been reported to have a higher incidence of angioedema compared to non-blacks. It should also be noted that in controlled clinical trials ACE inhibitors have an effect on blood pressure that is less in black patients than in non-blacks.
-
CONTRAINDICATIONSQuinapril is contraindicated in patients who are hypersensitive to this product and in patients with a history of angioedema related to previous treatment with an ACE inhibitor. Quinapril is ...Close
Quinapril is contraindicated in patients who are hypersensitive to this product and in patients with a history of angioedema related to previous treatment with an ACE inhibitor.
Quinapril is contraindicated in combination with a neprilysin inhibitor (e.g., sacubitril). Do not administer Quinapril within 36 hours of switching to or from sacubitril/valsartan, a neprilysin inhibitor (see WARNINGS and PRECAUTIONS).
Do not co-administer quinapril with aliskiren in patients with diabetes.
-
WARNINGSAnaphylactoid and Possibly Related Reactions - Presumably because ACE inhibitors affect the metabolism of eicosanoids and polypeptides, including endogenous bradykinin, patients receiving ACE ...Close
Anaphylactoid and Possibly Related Reactions
Presumably because ACE inhibitors affect the metabolism of eicosanoids and polypeptides, including endogenous bradykinin, patients receiving ACE inhibitors (including quinapril) may be subject to a variety of adverse reactions, some of them serious.
Head and Neck Angioedema: Angioedema of the face, extremities, lips, tongue, glottis, and larynx has been reported in patients treated with ACE inhibitors and has been seen in 0.1% of patients receiving quinapril.
In two similarly sized U.S. postmarketing trials that, combined, enrolled over 3,000 black patients and over 19,000 non-blacks, angioedema was reported in 0.30% and 0.55% of blacks (in study 1 and 2 respectively) and 0.39% and 0.17% of non-blacks.
Angioedema associated with laryngeal edema can be fatal. If laryngeal stridor or angioedema of the face, tongue, or glottis occurs, treatment with quinapril should be discontinued immediately, the patient treated in accordance with accepted medical care, and carefully observed until the swelling disappears. In instances where swelling is confined to the face and lips, the condition generally resolves without treatment; antihistamines may be useful in relieving symptoms. Where there is involvement of the tongue, glottis, or larynx likely to cause airway obstruction, emergency therapy including, but not limited to, subcutaneous epinephrine solution 1:1000 (0.3 to 0.5 mL) should be promptly administered (see ADVERSE REACTIONS).
Patients taking concomitant mammalian target of rapamycin (mTOR) inhibitor (e.g., temsirolimus) therapy or a neprilysin inhibitor may be at increased risk for angioedema.
Intestinal Angioedema: Intestinal angioedema has been reported in patients treated with ACE inhibitors. These patients presented with abdominal pain (with or without nausea or vomiting); in some cases there was no prior history of facial angioedema and C-1 esterase levels were normal. The angioedema was diagnosed by procedures including abdominal CT scan or ultrasound, or at surgery, and symptoms resolved after stopping the ACE inhibitor. Intestinal angioedema should be included in the differential diagnosis of patients on ACE inhibitors presenting with abdominal pain.
Patients with a history of angioedema: Patients with a history of angioedema unrelated to ACE inhibitor therapy may be at increased risk of angioedema while receiving an ACE inhibitor (see also CONTRAINDICATIONS).
Anaphylactoid reactions during desensitization: Two patients undergoing desensitizing treatment with hymenoptera venom while receiving ACE inhibitors sustained life-threatening anaphylactoid reactions. In the same patients, these reactions were avoided when ACE inhibitors were temporarily withheld, but they reappeared upon inadvertent rechallenge.
Anaphylactoid reactions during membrane exposure: Anaphylactoid reactions have been reported in patients dialyzed with high-flux membranes and treated concomitantly with an ACE inhibitor. Anaphylactoid reactions have also been reported in patients undergoing low-density lipoprotein apheresis with dextran sulfate absorption.
Hepatic Failure: Rarely, ACE inhibitors have been associated with a syndrome that starts with cholestatic jaundice and progresses to fulminant hepatic necrosis and (sometimes) death. The mechanism of this syndrome is not understood. Patients receiving ACE inhibitors who develop jaundice or marked elevations of hepatic enzymes should discontinue the ACE inhibitor and receive appropriate medical follow-up.
Hypotension: Excessive hypotension is rare in patients with uncomplicated hypertension treated with quinapril alone. Patients with heart failure given quinapril commonly have some reduction in blood pressure, but discontinuation of therapy because of continuing symptomatic hypotension usually is not necessary when dosing instructions are followed. Caution should be observed when initiating therapy in patients with heart failure (see DOSAGE AND ADMINISTRATION). In controlled studies, syncope was observed in 0.4% of patients (N=3203); this incidence was similar to that observed for captopril (1%) and enalapril (0.8%).
Patients at risk of excessive hypotension, sometimes associated with oliguria and/or progressive azotemia, and rarely with acute renal failure and/or death, include patients with the following conditions or characteristics: heart failure, hyponatremia, high dose diuretic therapy, recent intensive diuresis or increase in diuretic dose, renal dialysis, or severe volume and/or salt depletion of any etiology. It may be advisable to eliminate the diuretic (except in patients with heart failure), reduce the diuretic dose or cautiously increase salt intake (except in patients with heart failure) before initiating therapy with quinapril in patients at risk for excessive hypotension who are able to tolerate such adjustments.
In patients at risk of excessive hypotension, therapy with quinapril should be started under close medical supervision. Such patients should be followed closely for the first two weeks of treatment and whenever the dose of quinapril and/or diuretic is increased. Similar considerations may apply to patients with ischemic heart or cerebrovascular disease in whom an excessive fall in blood pressure could result in a myocardial infarction or a cerebrovascular accident.
If excessive hypotension occurs, the patient should be placed in the supine position and, if necessary, receive an intravenous infusion of normal saline. A transient hypotensive response is not a contraindication to further doses of quinapril, which usually can be given without difficulty once the blood pressure has stabilized. If symptomatic hypotension develops, a dose reduction or discontinuation of quinapril or concomitant diuretic may be necessary.
Neutropenia/Agranulocytosis: Another ACE inhibitor, captopril, has been shown to cause agranulocytosis and bone marrow depression rarely in patients with uncomplicated hypertension, but more frequently in patients with renal impairment, especially if they also have a collagen vascular disease, such as systemic lupus erythematosus or scleroderma. Agranulocytosis did occur during quinapril treatment in one patient with a history of neutropenia during previous captopril therapy. Available data from clinical trials of quinapril are insufficient to show that, in patients without prior reactions to other ACE inhibitors, quinapril does not cause agranulocytosis at similar rates. As with other ACE inhibitors, periodic monitoring of white blood cell counts in patients with collagen vascular disease and/or renal disease should be considered.
Fetal Toxicity
Pregnancy Category D
Use of drugs that act on the renin-angiotensin system during the second and third trimesters of pregnancy reduces fetal renal function and increases fetal and neonatal morbidity and death. Resulting oligohydramnios can be associated with fetal lung hypoplasia and skeletal deformations. Potential neonatal adverse effects include skull hypoplasia, anuria, hypotension, renal failure, and death. When pregnancy is detected, discontinue quinapril as soon as possible. These adverse outcomes are usually associated with use of these drugs in the second and third trimester of pregnancy. Most epidemiologic studies examining fetal abnormalities after exposure to antihypertensive use in the first trimester have not distinguished drugs affecting the renin-angiotensin system from other antihypertensive agents. Appropriate management of maternal hypertension during pregnancy is important to optimize outcomes for both mother and fetus.
In the unusual case that there is no appropriate alternative to therapy with drugs affecting the renin-angiotensin system for a particular patient, apprise the mother of the potential risk to the fetus. Perform serial ultrasound examinations to assess the intra-amniotic environment. If oligohydramnios is observed, discontinue quinapril, unless it is considered life-saving for the mother. Fetal testing may be appropriate, based on the week of pregnancy. Patients and physicians should be aware, however, that oligohydramnios may not appear until after the fetus has sustained irreversible injury. Closely observe infants with histories of in utero exposure to quinapril for hypotension, oliguria, and hyperkalemia (see PRECAUTIONS, Pediatric Use). No teratogenic effects of quinapril were seen in studies of pregnant rats and rabbits. On a mg/kg basis, the doses used were up to 180 times (in rats) and one time (in rabbits) the maximum recommended human dose.
-
PRECAUTIONSGeneral - Impaired renal function: As a consequence of inhibiting the renin-angiotensin-aldosterone system, changes in renal function may be anticipated in susceptible individuals. In ...Close
Impaired renal function: As a consequence of inhibiting the renin-angiotensin-aldosterone system, changes in renal function may be anticipated in susceptible individuals. In patients with severe heart failure whose renal function may depend on the activity of the renin-angiotensin-aldosterone system, treatment with ACE inhibitors, including quinapril, may be associated with oliguria and/or progressive azotemia and rarely acute renal failure and/or death.
In clinical studies in hypertensive patients with unilateral or bilateral renal artery stenosis, increases in blood urea nitrogen and serum creatinine have been observed in some patients following ACE inhibitor therapy. These increases were almost always reversible upon discontinuation of the ACE inhibitor and/or diuretic therapy. In such patients, renal function should be monitored during the first few weeks of therapy.
Some patients with hypertension or heart failure with no apparent preexisting renal vascular disease have developed increases in blood urea and serum creatinine, usually minor and transient, especially when quinapril has been given concomitantly with a diuretic. This is more likely to occur in patients with preexisting renal impairment. Dosage reduction and/or discontinuation of any diuretic and/or quinapril may be required.
Evaluation of patients with hypertension or heart failure should always include assessment of renal function (see DOSAGE AND ADMINISTRATION).
Hyperkalemia: In clinical trials, hyperkalemia (serum potassium ≥5.8 mmol/L) occurred in approximately 2% of patients receiving quinapril. In most cases, elevated serum potassium levels were isolated values which resolved despite continued therapy. Less than 0.1% of patients discontinued therapy due to hyperkalemia. Risk factors for the development of hyperkalemia include renal insufficiency, diabetes mellitus, and the concomitant use of other drugs that raise serum potassium levels. Monitor serum potassium in such patients (see PRECAUTIONS, Drug Interactions).
Cough: Presumably due to the inhibition of the degradation of endogenous bradykinin, persistent non-productive cough has been reported with all ACE inhibitors, always resolving after discontinuation of therapy. ACE inhibitor-induced cough should be considered in the differential diagnosis of cough.
Surgery/anesthesia: In patients undergoing major surgery or during anesthesia with agents that produce hypotension, quinapril will block angiotensin II formation secondary to compensatory renin release. If hypotension occurs and is considered to be due to this mechanism, it can be corrected by volume expansion.
Information for Patients
Pregnancy: Tell female patients of childbearing age about the consequences of exposure to quinapril during pregnancy. Discuss treatment options with women planning to become pregnant. Ask patients to report pregnancies to their physicians as soon as possible.
Angioedema: Angioedema, including laryngeal edema can occur with treatment with ACE inhibitors, especially following the first dose. Advise patients and tell them to immediately report any signs or symptoms suggesting angioedema (swelling of face, extremities, eyes, lips, tongue, difficulty in swallowing or breathing) and to stop taking the drug until they have consulted with their physician (see WARNINGS).
Symptomatic hypotension: Caution patients that lightheadedness can occur, especially during the first few days of quinapril therapy, and that it should be reported to a physician. If actual syncope occurs, tell patients to temporarily discontinue the drug until they have consulted with their physician (see WARNINGS).
Caution all patients that inadequate fluid intake or excessive perspiration, diarrhea, or vomiting can lead to an excessive fall in blood pressure because of reduction in fluid volume, with the same consequences of lightheadedness and possible syncope.
Tell patients planning to undergo any surgery and/or anesthesia to inform their physician that they are taking an ACE inhibitor.
Hyperkalemia: Tell patients not to use potassium supplements or salt substitutes containing potassium without consulting their physician (see PRECAUTIONS).
Neutropenia: Tell patients to promptly report any indication of infection (eg, sore throat, fever) which could be a sign of neutropenia.
NOTE: As with many other drugs, certain advice to patients being treated with quinapril is warranted. This information is intended to aid in the safe and effective use of this medication. It is not a disclosure of all possible adverse or intended effects.
Drug Interactions
Concomitant diuretic therapy: As with other ACE inhibitors, patients on diuretics, especially those on recently instituted diuretic therapy, may occasionally experience an excessive reduction of blood pressure after initiation of therapy with quinapril. The possibility of hypotensive effects with quinapril may be minimized by either discontinuing the diuretic or cautiously increasing salt intake prior to initiation of treatment with quinapril. If it is not possible to discontinue the diuretic, the starting dose of quinapril should be reduced (see DOSAGE AND ADMINISTRATION).
Agents increasing serum potassium: Coadministration of quinapril with other drugs that raise serum potassium levels may result in hyperkalemia. Monitor serum potassium in such patients.
Tetracycline and other drugs that interact with magnesium: Simultaneous administration of tetracycline with quinapril reduced the absorption of tetracycline by approximately 28% to 37%, possibly due to the high magnesium content in quinapril tablets. This interaction should be considered if coprescribing quinapril and tetracycline or other drugs that interact with magnesium.
Lithium: Increased serum lithium levels and symptoms of lithium toxicity have been reported in patients receiving concomitant lithium and ACE inhibitor therapy. These drugs should be coadministered with caution and frequent monitoring of serum lithium levels is recommended. If a diuretic is also used, it may increase the risk of lithium toxicity.
Gold: Nitritoid reactions (symptoms include facial flushing, nausea, vomiting, and hypotension) have been reported rarely in patients on therapy with injectable gold (sodium aurothiomalate) and concomitant ACE inhibitor therapy.
Non-steroidal anti-inflammatory agents including selective cyclooxygenase-2 inhibitors (COX-2 inhibitors): In patients who are elderly, volume-depleted (including those on diuretic therapy), or with compromised renal function, co-administration of NSAIDs, including selective COX-2 inhibitors, with ACE inhibitors, including quinapril, may result in deterioration of renal function, including possible acute renal failure. These effects are usually reversible. Monitor renal function periodically in patients receiving quinapril and NSAID therapy.
The antihypertensive effect of ACE inhibitors, including quinapril may be attenuated by NSAIDs.
Agents that inhibit mTOR or other drugs known to cause angioedema: Patients taking concomitant mTOR inhibitor (e.g. temsirolimus) therapy or a neprilysin inhibitor may be at increased risk for angioedema.
Other agents: Drug interaction studies of quinapril with other agents showed:
- Multiple dose therapy with propranolol or cimetidine has no effect on the pharmacokinetics of single doses of quinapril.
- The anticoagulant effect of a single dose of warfarin (measured by prothrombin time) was not significantly changed by quinapril coadministration twice-daily.
- Quinapril treatment did not affect the pharmacokinetics of digoxin.
- No pharmacokinetic interaction was observed when single doses of quinapril and hydrochlorothiazide were administered concomitantly.
- Co-administration of multiple 10 mg doses of atorvastatin with 80 mg of quinapril resulted in no significant change in the steady-state pharmacokinetic parameters of atorvastatin.
Dual Blockade of the Renin-Angiotensin System (RAS): Dual blockade of the RAS with angiotensin receptor blockers, ACE inhibitors, or aliskiren is associated with increased risks of hypotension, hyperkalemia, and changes in renal function (including acute renal failure) compared to monotherapy. Most patients receiving the combination of two RAS inhibitors do not obtain any additional benefit compared to monotherapy. In general, avoid combined use of RAS inhibitors. Closely monitor blood pressure, renal function and electrolytes in patients on quinapril and other agents that affect the RAS.
Do not co-administer aliskiren with quinapril in patients with diabetes. Avoid concomitant use of aliskiren with quinapril in patients with renal impairment (GFR<60 mL/min/1.73 m2).
Carcinogenesis, Mutagenesis, Impairment of Fertility
Quinapril hydrochloride was not carcinogenic in mice or rats when given in doses up to 75 or 100 mg/kg/day (50 to 60 times the maximum human daily dose, respectively, on an mg/kg basis and 3.8 to 10 times the maximum human daily dose when based on an mg/m2basis) for 104 weeks. Female rats given the highest dose level had an increased incidence of mesenteric lymph node hemangiomas and skin/subcutaneous lipomas. Neither quinapril nor quinaprilat were mutagenic in the Ames bacterial assay with or without metabolic activation. Quinapril was also negative in the following genetic toxicology studies: in vitro mammalian cell point mutation, sister chromatid exchange in cultured mammalian cells, micronucleus test with mice, in vitro chromosome aberration with V79 cultured lung cells, and in an in vivo cytogenetic study with rat bone marrow. There were no adverse effects on fertility or reproduction in rats at doses up to 100 mg/kg/day (60 and 10 times the maximum daily human dose when based on mg/kg and mg/m2, respectively).
Nursing Mothers
Because quinapril is secreted in human milk, caution should be exercised when this drug is administered to a nursing woman.
Pediatric Use
Neonates with a history of in utero exposure to quinapril:
If oliguria or hypotension occurs, direct attention toward support of blood pressure and renal perfusion. Exchange transfusions or dialysis may be required as a means of reversing hypotension and/or substituting for disordered renal function. Removal of quinapril, which crosses the placenta, from the neonatal circulation is not significantly accelerated by these means.
The safety and effectiveness of quinapril in pediatric patients have not been established.
Geriatric Use
Clinical studies of quinapril did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal or cardiac function, and of concomitant disease or other drug therapy.
This drug is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function.
Elderly patients exhibited increased area under the plasma concentration time curve and peak levels for quinaprilat compared to values observed in younger patients; this appeared to relate to decreased renal function rather than to age itself.
-
ADVERSE REACTIONSHypertension - Quinapril has been evaluated for safety in 4960 subjects and patients. Of these, 3203 patients, including 655 elderly patients, participated in controlled clinical trials ...Close
Quinapril has been evaluated for safety in 4960 subjects and patients. Of these, 3203 patients, including 655 elderly patients, participated in controlled clinical trials. Quinapril has been evaluated for long-term safety in over 1400 patients treated for 1 year or more.
Adverse experiences were usually mild and transient.
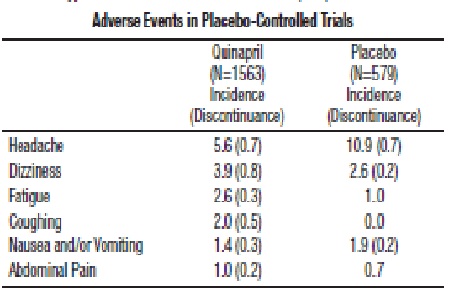
In placebo-controlled trials, discontinuation of therapy because of adverse events was required in 4.7% of patients with hypertension.
Adverse experiences probably or possibly related to therapy or of unknown relationship to therapy occurring in 1% or more of the 1563 patients in placebo-controlled hypertension trials who were treated with quinapril are shown below.
Quinapril has been evaluated for safety in 1222 quinapril treated patients. Of these, 632 patients participated in controlled clinical trials. In placebo-controlled trials, discontinuation of therapy because of adverse events was required in 6.8% of patients with congestive heart failure.
Adverse experiences probably or possibly related or of unknown relationship to therapy occurring in 1% or more of the 585 patients in placebo-controlled congestive heart failure trials who were treated with quinapril are shown below.
Quinapril
(N=585)
Incidence
(Discontinuance)Placebo
(N=295)
Incidence
(Discontinuance)Dizziness 7.7 (0.7) 5.1 (1.0) Coughing 4.3 (0.3) 1.4 Fatigue 2.6 (0.2) 1.4 Nausea and/or Vomiting 2.4 (0.2) 0.7 Chest Pain 2.4 1.0 Hypotension 2.9 (0.5) 1.0 Dyspnea 1.9 (0.2) 2.0 Diarrhea 1.7 1.0 Headache 1.7 1.0 (0.3) Myalgia 1.5 2.0 Rash 1.4 (0.2) 1.0 Back Pain 1.2 0.3 See PRECAUTIONS, Cough.
Hypertension and/or Heart Failure
Clinical adverse experiences probably, possibly, or definitely related, or of uncertain relationship to therapy occurring in 0.5% to 1.0% (except as noted) of the patients with CHF or hypertension treated with quinapril (with or without concomitant diuretic) in controlled or uncontrolled trials (N=4847) and less frequent, clinically significant events seen in clinical trials or post-marketing experience (the rarer events are in italics) include (listed by body system):
General: back pain, malaise, viral infections, anaphylactoid reaction
Cardiovascular: palpitation, vasodilation, tachycardia, heart failure, hyperkalemia, myocardial infarction, cerebrovascular accident, hypertensive crisis, angina pectoris, orthostatic hypotension, cardiac rhythm disturbances, cardiogenic shock
Hematology: hemolytic anemia
Gastrointestinal: flatulence, dry mouth or throat, constipation, gastrointestinal hemorrhage, pancreatitis, abnormal liver function tests, dyspepsia
Metabolism and Nutrition Disorders: hyponatremia
Nervous/Psychiatric: somnolence, vertigo, syncope, nervousness, depression, insomnia, paresthesia
Integumentary: alopecia, increased sweating, pemphigus, pruritus, exfoliative dermatitis, photosensitivity reaction, dermatopolymyositis
Urogenital: urinary tract infection, impotence, acute renal failure, worsening renal failure
Respiratory: eosinophilic pneumonitis
Other: amblyopia, edema, arthralgia, pharyngitis, agranulocytosis, hepatitis, thrombocytopenia
Angioedema
Angioedema has been reported in patients receiving quinapril (0.1%). Angioedema associated with laryngeal edema may be fatal. If angioedema of the face, extremities, lips, tongue, glottis, and/or larynx occurs, treatment with quinapril should be discontinued and appropriate therapy instituted immediately. (See WARNINGS)
Clinical Laboratory Test Findings
Hematology: (See WARNINGS)
Hyperkalemia: (See PRECAUTIONS)
Creatinine and Blood Urea Nitrogen: Increases (>1.25 times the upper limit of normal) in serum creatinine and blood urea nitrogen were observed in 2% and 2%, respectively, of all patients treated with quinapril alone. Increases are more likely to occur in patients receiving concomitant diuretic therapy than in those on quinapril alone. These increases often remit on continued therapy. In controlled studies of heart failure, increases in blood urea nitrogen and serum creatinine were observed in 11% and 8%, respectively, of patients treated with quinapril; most often these patients were receiving diuretics with or without digitalis.
-
OVERDOSAGEDoses of 1440 to 4280 mg/kg of quinapril cause significant lethality in mice and rats. No specific information is available on the treatment of overdosage with quinapril. The most likely ...Close
Doses of 1440 to 4280 mg/kg of quinapril cause significant lethality in mice and rats.
No specific information is available on the treatment of overdosage with quinapril. The most likely clinical manifestation would be symptoms attributable to severe hypotension.
Laboratory determinations of serum levels of quinapril and its metabolites are not widely available, and such determinations have, in any event, no established role in the management of quinapril overdose.
No data are available to suggest physiological maneuvers (eg, maneuvers to change pH of the urine) that might accelerate elimination of quinapril and its metabolites.
Hemodialysis and peritoneal dialysis have little effect on the elimination of quinapril and quinaprilat. Angiotensin II could presumably serve as a specific antagonist-antidote in the setting of quinapril overdose, but angiotensin II is essentially unavailable outside of scattered research facilities. Because the hypotensive effect of quinapril is achieved through vasodilation and effective hypovolemia, it is reasonable to treat quinapril overdose by infusion of normal saline solution.
-
DOSAGE AND ADMINISTRATIONHypertension - Monotherapy: The recommended initial dosage of quinapril in patients not on diuretics is 10 or 20 mg once daily. Dosage should be adjusted according to blood pressure response ...Close
Monotherapy: The recommended initial dosage of quinapril in patients not on diuretics is 10 or 20 mg once daily. Dosage should be adjusted according to blood pressure response measured at peak (2 to 6 hours after dosing) and trough (predosing). Generally, dosage adjustments should be made at intervals of at least 2 weeks. Most patients have required dosages of 20, 40, or 80 mg/day, given as a single dose or in two equally divided doses. In some patients treated once daily, the antihypertensive effect may diminish toward the end of the dosing interval. In such patients an increase in dosage or twice daily administration may be warranted. In general, doses of 40–80 mg and divided doses give a somewhat greater effect at the end of the dosing interval.
Concomitant Diuretics: If blood pressure is not adequately controlled with quinapril monotherapy, a diuretic may be added. In patients who are currently being treated with a diuretic, symptomatic hypotension occasionally can occur following the initial dose of quinapril. To reduce the likelihood of hypotension, the diuretic should, if possible, be discontinued 2 to 3 days prior to beginning therapy with quinapril (see WARNINGS). Then, if blood pressure is not controlled with quinapril alone, diuretic therapy should be resumed.
If the diuretic cannot be discontinued, an initial dose of 5 mg quinapril should be used with careful medical supervision for several hours and until blood pressure has stabilized.
The dosage should subsequently be titrated (as described above) to the optimal response (see WARNINGS, PRECAUTIONS, and Drug Interactions).
Renal Impairment: Kinetic data indicate that the apparent elimination half-life of quinaprilat increases as creatinine clearance decreases. Recommended starting doses, based on clinical and pharmacokinetic data from patients with renal impairment, are as follows:
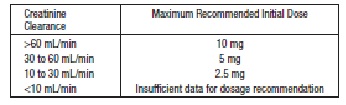
Patients should subsequently have their dosage titrated (as described above) to the optimal response.
Elderly (≥65 years): The recommended initial dosage of quinapril in elderly patients is 10 mg given once daily followed by titration (as described above) to the optimal response.
Heart Failure
Quinapril is indicated as adjunctive therapy when added to conventional therapy including diuretics and/or digitalis. The recommended starting dose is 5 mg twice daily. This dose may improve symptoms of heart failure, but increases in exercise duration have generally required higher doses. Therefore, if the initial dosage of quinapril is well tolerated, patients should then be titrated at weekly intervals until an effective dose, usually 20 to 40 mg daily given in two equally divided doses, is reached or undesirable hypotension, orthostatis, or azotemia (see WARNINGS) prohibit reaching this dose.
Following the initial dose of quinapril, the patient should be observed under medical supervision for at least two hours for the presence of hypotension or orthostatis and, if present, until blood pressure stabilizes. The appearance of hypotension, orthostatis, or azotemia early in dose titration should not preclude further careful dose titration. Consideration should be given to reducing the dose of concomitant diuretics.
DOSE ADJUSTMENTS IN PATIENTS WITH HEART FAILURE AND RENAL IMPAIRMENT OR HYPONATREMIA
Pharmacokinetic data indicate that quinapril elimination is dependent on level of renal function. In patients with heart failure and renal impairment, the recommended initial dose of quinapril is 5 mg in patients with a creatinine clearance above 30 mL/min and 2.5 mg in patients with a creatinine clearance of 10 to 30 mL/min. There is insufficient data for dosage recommendation in patients with a creatinine clearance less than 10 mL/min (see DOSAGE AND ADMINISTRATION, Heart Failure, WARNINGS, and PRECAUTIONS, Drug Interactions).
If the initial dose is well tolerated, quinapril may be administered the following day as a twice daily regimen. In the absence of excessive hypotension or significant deterioration of renal function, the dose may be increased at weekly intervals based on clinical and hemodynamic response.
-
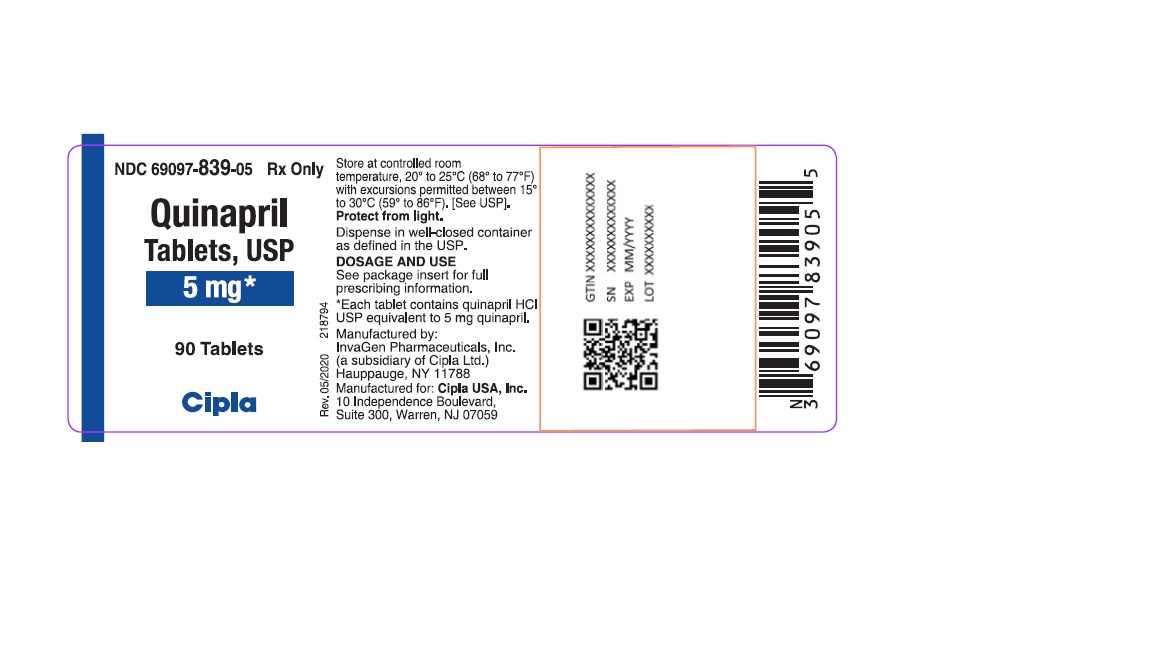
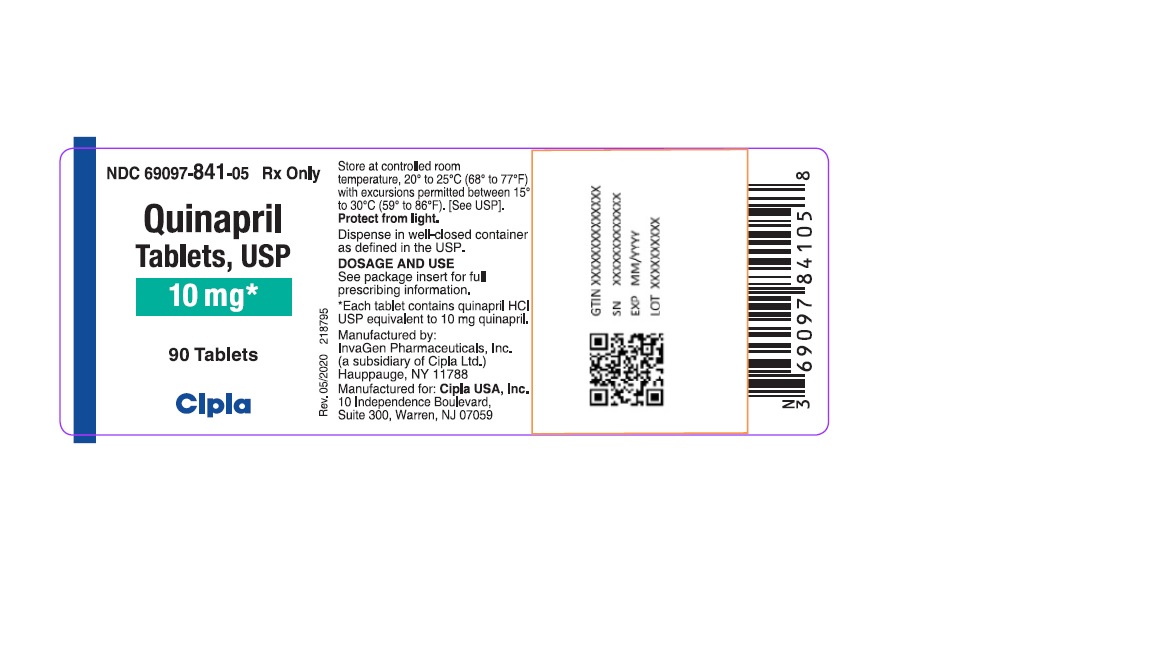
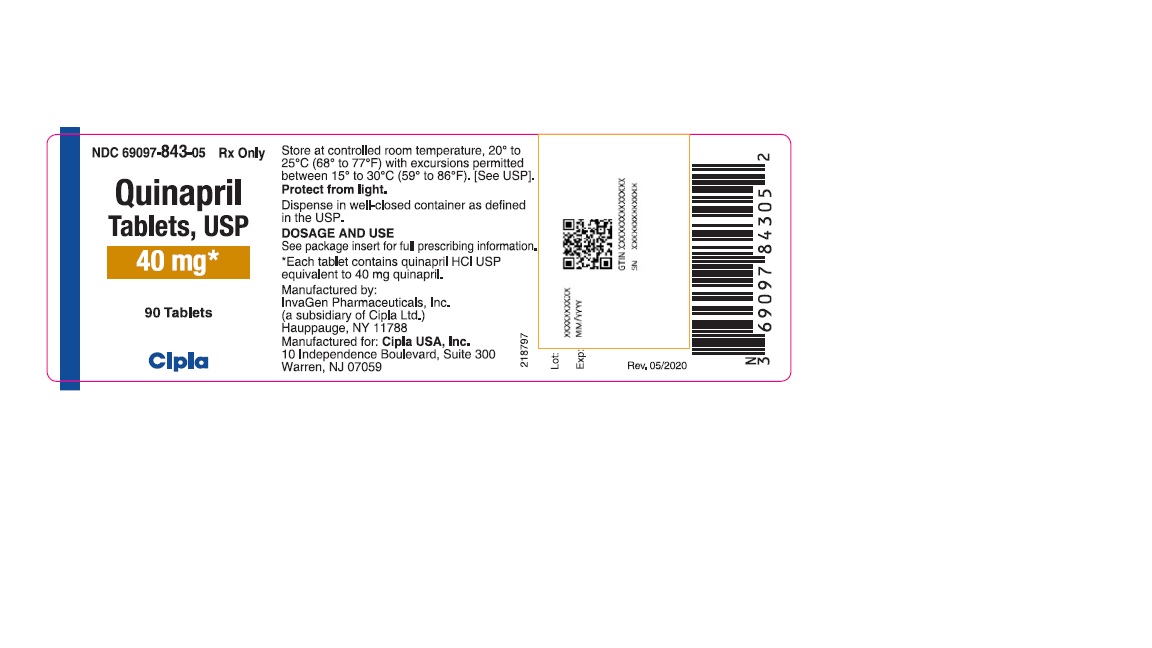
HOW SUPPLIEDQuinapril tablets, USP are supplied as follows: 5-mg tablets: brown, round biconvex tablets de-bossed with I on the left side of bisect and G on the right side of bisect and 267 on other. NDC ...Close
Quinapril tablets, USP are supplied as follows:
5-mg tablets: brown, round biconvex tablets de-bossed with I on the left side of bisect and G on the right side of bisect and 267 on other.
NDC 69097-839-05 bottles of 90 tablets
NDC 69097-839-15 bottles of 1000 tablets
10-mg tablets: brown, round biconvex tablets de-bossed with IG on one side and 268 on other.
NDC 69097-841-05 bottles of 90 tablets
NDC 69097-841-15 bottles of 1000 tablets
20-mg tablets: brown, round biconvex tablets de-bossed with IG on one side and 269 on other.
NDC 69097-842-05 bottles of 90 tablets
NDC 69097-842-15 bottles of 1000 tablets
40-mg tablets: brown, oval biconvex tablets de-bossed with IG on one side and 270 on other.
NDC 69097-843-05 bottles of 90 tablets
NDC 69097-843-15 bottles of 1000 tablets
Dispense in well-closed containers as defined in the USP.
Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature]
Protect from light.
Manufactured by:
InvaGen Pharmaceuticals, Inc.
(a subsidiary of Cipla Ltd.)
Hauppauge, NY 11788
Manufactured for:
Cipla USA Inc.
10 Independence Boulevard, Suite 300
Warren, NJ 07059
Rev: 05/2020
SAP code: 21083900
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANELNDC 69097-839-05 Rx Only - Quinapril Tablets, USP - 5 mg* 90 Tablets - Cipla - NDC 69097-841-05 Rx Only - Quinapril Tablets, USP - 10 mg* 90 Tablets - Cipla - NDC 69097-842-05 Rx Only - Quinapril Tablets ...
Quinapril Tablets, USP
5 mg*
90 Tablets
Cipla
Quinapril Tablets, USP
10 mg*
90 Tablets
Cipla
Quinapril Tablets, USP
20 mg*
90 Tablets
Cipla

Quinapril Tablets, USP
40 mg*
90 Tablets
Cipla
Close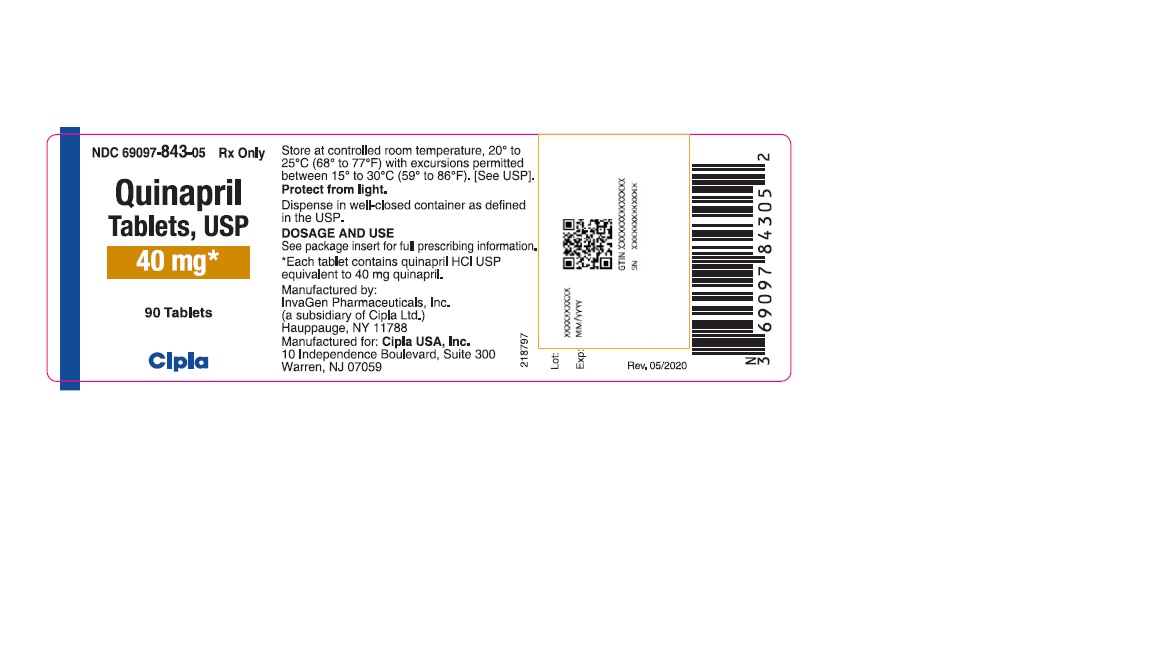
-
INGREDIENTS AND APPEARANCEProduct Information
QUINAPRIL quinapril tablet Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:69097-839 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength QUINAPRIL HYDROCHLORIDE (UNII: 33067B3N2M) (QUINAPRILAT - UNII:34SSX5LDE5) QUINAPRIL 5 mg Inactive Ingredients Ingredient Name Strength LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM CARBONATE (UNII: 0E53J927NA) MAGNESIUM STEARATE (UNII: 70097M6I30) CROSPOVIDONE, UNSPECIFIED (UNII: 2S7830E561) POVIDONE, UNSPECIFIED (UNII: FZ989GH94E) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) Product Characteristics Color BROWN Score 2 pieces Shape ROUND (Biconvex) Size 6mm Flavor Imprint Code IG;267 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69097-839-05 90 in 1 BOTTLE; Type 0: Not a Combination Product 07/22/2016 2 NDC:69097-839-15 1000 in 1 BOTTLE; Type 0: Not a Combination Product 07/22/2016 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA078457 07/22/2016 QUINAPRIL quinapril tablet Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:69097-841 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength QUINAPRIL HYDROCHLORIDE (UNII: 33067B3N2M) (QUINAPRILAT - UNII:34SSX5LDE5) QUINAPRIL 10 mg Inactive Ingredients Ingredient Name Strength LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM CARBONATE (UNII: 0E53J927NA) MAGNESIUM STEARATE (UNII: 70097M6I30) CROSPOVIDONE, UNSPECIFIED (UNII: 2S7830E561) POVIDONE, UNSPECIFIED (UNII: FZ989GH94E) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) Product Characteristics Color BROWN Score no score Shape ROUND (Biconvex) Size 6mm Flavor Imprint Code IG;268 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69097-841-05 90 in 1 BOTTLE; Type 0: Not a Combination Product 07/22/2016 2 NDC:69097-841-15 1000 in 1 BOTTLE; Type 0: Not a Combination Product 07/22/2016 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA078457 07/22/2016 QUINAPRIL quinapril tablet Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:69097-842 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength QUINAPRIL HYDROCHLORIDE (UNII: 33067B3N2M) (QUINAPRILAT - UNII:34SSX5LDE5) QUINAPRIL 20 mg Inactive Ingredients Ingredient Name Strength LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM CARBONATE (UNII: 0E53J927NA) MAGNESIUM STEARATE (UNII: 70097M6I30) CROSPOVIDONE, UNSPECIFIED (UNII: 2S7830E561) POVIDONE, UNSPECIFIED (UNII: FZ989GH94E) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) Product Characteristics Color BROWN Score no score Shape ROUND (Biconvex) Size 8mm Flavor Imprint Code IG;269 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69097-842-05 90 in 1 BOTTLE; Type 0: Not a Combination Product 07/22/2016 2 NDC:69097-842-15 1000 in 1 BOTTLE; Type 0: Not a Combination Product 07/22/2016 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA078457 07/22/2016 QUINAPRIL quinapril tablet Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:69097-843 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength QUINAPRIL HYDROCHLORIDE (UNII: 33067B3N2M) (QUINAPRILAT - UNII:34SSX5LDE5) QUINAPRIL 40 mg Inactive Ingredients Ingredient Name Strength LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM CARBONATE (UNII: 0E53J927NA) MAGNESIUM STEARATE (UNII: 70097M6I30) CROSPOVIDONE, UNSPECIFIED (UNII: 2S7830E561) POVIDONE, UNSPECIFIED (UNII: FZ989GH94E) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) Product Characteristics Color BROWN Score no score Shape OVAL (Biconvex) Size 14mm Flavor Imprint Code IG;270 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69097-843-05 90 in 1 BOTTLE; Type 0: Not a Combination Product 07/22/2016 2 NDC:69097-843-15 1000 in 1 BOTTLE; Type 0: Not a Combination Product 07/22/2016 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA078457 07/22/2016 Labeler - Cipla USA Inc. (078719707) Registrant - Cipla USA Inc. (078719707) Establishment Name Address ID/FEI Business Operations InvaGen Pharmaceutical Inc 165104469 analysis(69097-839, 69097-841, 69097-842, 69097-843) , manufacture(69097-839, 69097-841, 69097-842, 69097-843)
CloseEstablishment Name Address ID/FEI Business Operations InvaGen Pharmaceutical Inc 080334903 analysis(69097-839, 69097-841, 69097-842, 69097-843) , pack(69097-839, 69097-841, 69097-842, 69097-843)
Find additional resources
(also available in the left menu)Safety
Boxed Warnings, Report Adverse Events, FDA Safety Recalls, Presence in Breast Milk
Related Resources
Medline Plus, Clinical Trials, PubMed, Biochemical Data Summary
More Info on this Drug
View Labeling Archives, RxNorm, Get Label RSS Feed, View NDC Code(s)NEW!
RxNorm
QUINAPRIL tablet
| RxCUI | RxNorm NAME | RxTTY | |
|---|---|---|---|
| 1 | 312748 | quinapril HCl 10 MG Oral Tablet | PSN |
| 2 | 312748 | quinapril 10 MG Oral Tablet | SCD |
| 3 | 312748 | quinapril (as quinapril HCl) 10 MG Oral Tablet | SY |
| 4 | 312749 | quinapril HCl 20 MG Oral Tablet | PSN |
| 5 | 312749 | quinapril 20 MG Oral Tablet | SCD |
| 6 | 312749 | quinapril (as quinapril HCl) 20 MG Oral Tablet | SY |
| 7 | 312750 | quinapril HCl 5 MG Oral Tablet | PSN |
| 8 | 312750 | quinapril 5 MG Oral Tablet | SCD |
| 9 | 312750 | quinapril (as quinapril hydrochloride) 5 MG Oral Tablet | SY |
| 10 | 314203 | quinapril HCl 40 MG Oral Tablet | PSN |
| 11 | 314203 | quinapril 40 MG Oral Tablet | SCD |
| 12 | 314203 | quinapril (as quinapril hydrochloride) 40 MG Oral Tablet | SY |
Get Label RSS Feed for this Drug
QUINAPRIL tablet
To receive this label RSS feed
Copy the URL below and paste it into your RSS Reader application.
https://dailymed.nlm.nih.gov/dailymed/labelrss.cfm?setid=e6d36e1e-8e35-4d06-83dc-45e0456365fd
To receive all DailyMed Updates for the last seven days
Copy the URL below and paste it into your RSS Reader application.
https://dailymed.nlm.nih.gov/dailymed/rss.cfm
What will I get with the DailyMed RSS feed?
DailyMed will deliver notification of updates and additions to Drug Label information currently shown on this site through its RSS feed.
DailyMed will deliver this notification to your desktop, Web browser, or e-mail depending on the RSS Reader you select to use. To view updated drug label links, paste the RSS feed address (URL) shown below into a RSS reader, or use a browser which supports RSS feeds, such as Safari for Mac OS X.
How to discontinue the RSS feed
If you no longer wish to have this DailyMed RSS service, simply delete the copied URL from your RSS Reader.
More about getting RSS News & Updates from DailyMedWhy is DailyMed no longer displaying pill images on the Search Results and Drug Info pages?
Due to inconsistencies between the drug labels on DailyMed and the pill images provided by RxImage, we no longer display the RxImage pill images associated with drug labels.
We anticipate reposting the images once we are able identify and filter out images that do not match the information provided in the drug labels.
NDC Codes
QUINAPRIL tablet
If this SPL contains inactivated NDCs listed by the FDA initiated compliance action, they will be specified as such.
| NDC | |
|---|---|
| 1 | 69097-839-05 |
| 2 | 69097-839-15 |
| 3 | 69097-841-05 |
| 4 | 69097-841-15 |
| 5 | 69097-842-05 |
| 6 | 69097-842-15 |
| 7 | 69097-843-05 |
| 8 | 69097-843-15 |