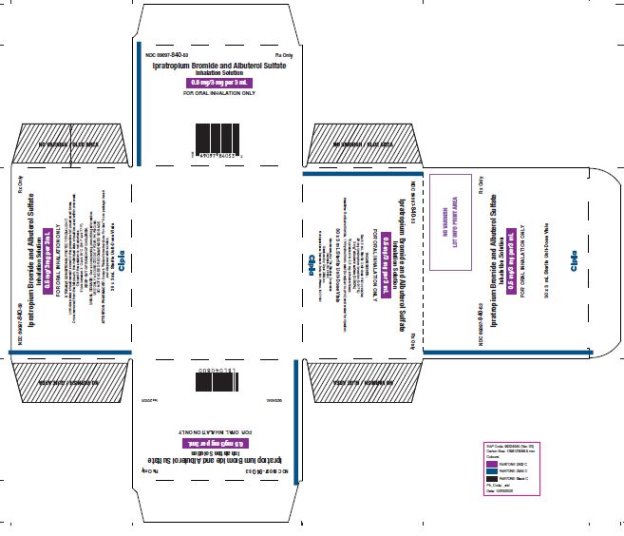
Ipratropium Bromide and Albuterol Sulfate Inhalation Solution
-
0.5 mg / 3 mg per 3 mL
-
Prescription Only.
Read the patient information that comes with Ipratropium Bromide 0.5 mg and ...
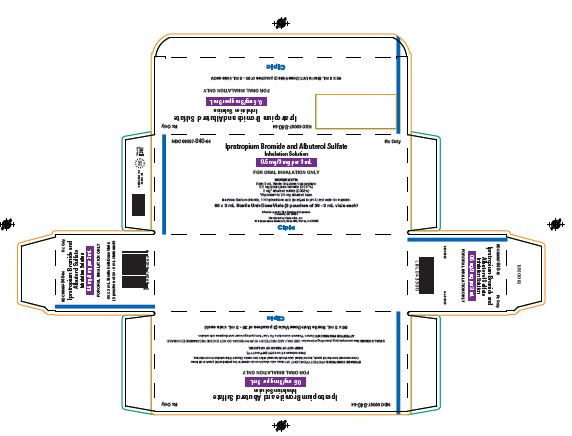
Ipratropium Bromide and Albuterol Sulfate Inhalation Solution
0.5 mg / 3 mg per 3 mL
Prescription Only.
Read the patient information that comes with Ipratropium Bromide 0.5 mg and Albuterol Sulfate 3 mg before you start using it and each time you get a refill. There may be new information. This leaflet does not take the place of talking with your doctor about your medical condition or your treatment.
What is Ipratropium Bromide 0.5 mg and Albuterol Sulfate 3 mg?
Ipratropium Bromide 0.5 mg and Albuterol Sulfate 3 mg is a combination of two medicines called bronchodilators. Ipratropium Bromide 0.5 mg and Albuterol Sulfate 3 mg contains albuterol sulfate, which is a beta-adrenergic agonist, and ipratropium bromide, which is an anticholinergic. These two medicines work together to help open the airways in your lungs. Ipratropium Bromide 0.5 mg and Albuterol Sulfate 3 mg is used to help treat airway narrowing (bronchospasm) that happens with chronic obstructive pulmonary disease (COPD) in adult patients who need to use more than one bronchodilator medicine.
Who should not use Ipratropium Bromide 0.5 mg and Albuterol Sulfate 3 mg?
Do not use Ipratropium Bromide 0.5 mg and Albuterol Sulfate 3 mg if you: Are allergic to any of the ingredients in Ipratropium Bromide 0.5 mg and Albuterol Sulfate 3 mg or to atropine. The active ingredients are albuterol sulfate and ipratropium bromide. See the end of this leaflet for a complete list of ingredients in Ipratropium Bromide 0.5 mg and Albuterol Sulfate 3 mg
Ipratropium Bromide 0.5 mg and Albuterol Sulfate 3 mg has not been studied in patients younger than 18 years of age.
What should I tell my doctor before I start using Ipratropium Bromide 0.5 mg and Albuterol Sulfate 3 mg?
Tell your doctor about all of your conditions, including if you:
- Have heart problems. This includes coronary artery disease and heart rhythm problems.
- Have high blood pressure
- Have diabetes
- Have or had seizures
- Have a thyroid problem called hyperthyroidism
- Have an eye problem called narrow-angle glaucoma
- Have liver or kidney problems
- Have problems urinating due to bladder-neck blockage or an enlarged prostate (men)
- Are pregnant or planning to become pregnant. It is not known if Ipratropium Bromide 0.5 mg and Albuterol Sulfate 3 mg can harm your unborn baby. You and your doctor will have to decide if Ipratropium Bromide 0.5 mg and Albuterol Sulfate 3 mg is right for you during a pregnancy.
- Are breastfeeding. It is not known if Ipratropium Bromide 0.5 mg and Albuterol Sulfate 3 mg passes into your milk or if it can harm your baby. You and your doctor should decide whether you should take Ipratropium Bromide 0.5 mg and Albuterol Sulfate 3 mg or breastfeed, but not both.
Tell your doctor about all the medicines you take including prescription and non-prescription medicines, vitamins and herbal supplements. Ipratropium Bromide 0.5 mg and Albuterol Sulfate 3 mg and other medicines can interact. This may cause serious side effects. Especially tell your doctor if you take:
- Other medicines that contain anticholinergics such as ipratropium bromide. This also includes medicines used for Parkinson's disease.
- Other medicines that contain beta-agonists such as albuterol sulfate. These are usually used to treat airway narrowing (bronchospasm).
- Medicines called beta-blockers. These are usually used for high blood pressure or heart problems.
- Medicines called "water pills" (diuretics)
- Medicines for depression called monoamine oxidase inhibitors (MAOIs) or tricyclic antidepressants.
Ask your doctor or pharmacist if you are not sure if you take any of these types of medicines. Know the medicines you take. Keep a list of them and show it to your doctor and pharmacists when you get a new medicine.
How should I use Ipratropium Bromide 0.5 mg and Albuterol Sulfate 3 mg?
- Read the Patient's Instructions for Use that you get with your prescription. Talk to your doctor or pharmacist if you have any questions.
- Take Ipratropium Bromide 0.5 mg and Albuterol Sulfate 3 mg exactly as prescribed by your doctor. Do not change your dose or how often you use Ipratropium Bromide 0.5 mg and Albuterol Sulfate 3 mg without talking to your doctor. Inhale Ipratropium Bromide 0.5 mg and Albuterol Sulfate 3 mg through your mouth and into your lungs using a machine called a nebulizer.
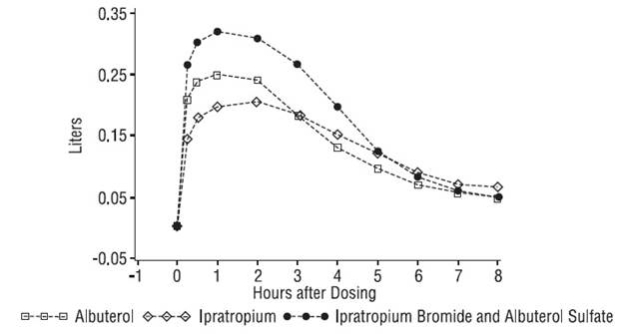
- Ipratropium Bromide 0.5 mg and Albuterol Sulfate 3 mg may help to open your airways for up to 5 hours after taking this medicine. If Ipratropium Bromide 0.5 mg and Albuterol Sulfate 3 mg does not help your airway narrowing (bronchospasm) or your bronchospasm gets worse, call your doctor right away or get emergency help if needed.
What should I avoid while using Ipratropium Bromide 0.5 mg and Albuterol Sulfate 3 mg?
Do not get Ipratropium Bromide 0.5 mg and Albuterol Sulfate 3 mg in your eyes. Be careful not to spray Ipratropium Bromide 0.5 mg and Albuterol Sulfate 3 mg in your eyes while you are using your nebulizer. Ipratropium Bromide 0.5 mg and Albuterol Sulfate 3 mg can cause the following short-term eye problems:
- Enlarged pupils
- Blurry vision
- Eye pain
Ipratropium Bromide 0.5 mg and Albuterol Sulfate 3 mg can cause a serious eye problem called narrow-angle glaucoma or worsen the narrow-angle glaucoma you already have.
What are the possible side effects with Ipratropium Bromide 0.5 mg and Albuterol Sulfate 3 mg?
Ipratropium Bromide 0.5 mg and Albuterol Sulfate 3 mg may cause the following serious side effects:
-
Worsening of the narrowing in your airways (bronchospasm). This side effect can be life-threatening and has happened with both of the medicines that are in Ipratropium Bromide 0.5 mg and Albuterol Sulfate 3 mg. Stop Ipratropium Bromide 0.5 mg and Albuterol Sulfate 3 mg and call your doctor right away or get emergency help if your breathing problems get worse while or after using Ipratropium Bromide 0.5 mg and Albuterol Sulfate 3 mg
-
Serious and life-threatening allergic reactions. Symptoms of a serious allergic reaction include:
- Hives, rash
- Swelling of your face, eyelids, lips, tongue, or throat, and trouble swallowing
- Worsening of your breathing problems such as wheezing, chest tightness or shortness of breath
- Shock (loss of blood pressure and consciousness)
The most common side effects with Ipratropium Bromide 0.5 mg and Albuterol Sulfate 3 mg include lung disease, sore throat, chest pain, constipation, diarrhea, bronchitis, urinary tract infection, leg cramps, nausea, upset stomach, voice changes, and pain.
These are not all the side effects with Ipratropium Bromide 0.5 mg and Albuterol Sulfate 3 mg. For a complete list, ask your doctor or pharmacist.
How should I store Ipratropium Bromide 0.5 mg and Albuterol Sulfate 3 mg?
- Store Ipratropium Bromide 0.5 mg and Albuterol Sulfate 3 mg between 36° and 77°F (2° and 25°C). Protect from light. Keep the unused vials in the foil pouch or carton.
- Safely discard Ipratropium Bromide 0.5 mg and Albuterol Sulfate 3 mg that is out-of-date or no longer needed.
- Keep Ipratropium Bromide 0.5 mg and Albuterol Sulfate 3 mg and all medicines out of the reach of children.
General advice about Ipratropium Bromide 0.5 mg and Albuterol Sulfate 3 mg
Medicines are sometimes prescribed for conditions that are not mentioned in the patient information leaflets. Do not use Ipratropium Bromide 0.5 mg and Albuterol Sulfate 3 mg for a condition for which it was not prescribed. Do not give Ipratropium Bromide 0.5 mg and Albuterol Sulfate 3 mg to other people, even if they have the same symptoms you have. It may harm them.
This leaflet summarizes the most important information about Ipratropium Bromide 0.5 mg and Albuterol Sulfate 3 mg. If you would like more information, talk with your doctor. You can ask your doctor or pharmacist for information about Ipratropium Bromide 0.5 mg and Albuterol Sulfate 3 mg that is written for healthcare professionals.
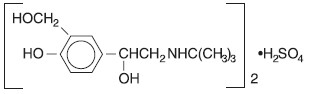
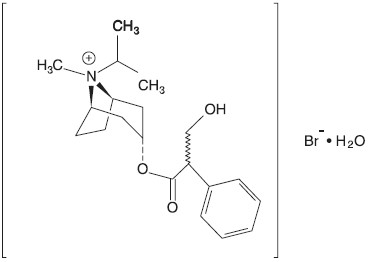
What are the ingredients in Ipratropium Bromide 0.5 mg and Albuterol Sulfate 3 mg?
Active Ingredients: ipratropium bromide and albuterol sulfate
Inactive Ingredients: sodium chloride and 1 N hydrochloric acid
To report SUSPECTED ADVERSE REACTIONS, contact The Ritedose Corporation at 1-855-806-3300 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Manufactured By:
The Ritedose Corporation
Columbia, SC 29203 for
Ritedose Pharmaceuticals, LLC
Columbia, SC 29203
Distributed By:
Cipla USA Inc.
10 Independence Boulevard , Suite 300,
Warren, NJ 07059
9/2020
Close