Label: TERBINAFINE HYDROCHLORIDE- terbinafine tablets 250 mg tablet
- NDC Code(s): 69097-731-02, 69097-731-07
- Packager: Cipla USA Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated January 5, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use TERBINAFINE TABLETS safely and effectively. See full prescribing information for TERBINAFINE TABLETS. TERBINAFINE tablets,for oral ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGETerbinafine tablets are indicated for the treatment of onychomycosis of the toenail or fingernail due to dermatophytes (tinea unguium). Prior to initiating treatment, appropriate nail ...
-
2 DOSAGE AND ADMINISTRATION2.1 Assessment Prior to Initiation - Before administering terbinafine tablets, evaluate patients for evidence of chronic or active liver disease [see Contraindications (4) and Warnings and ...
-
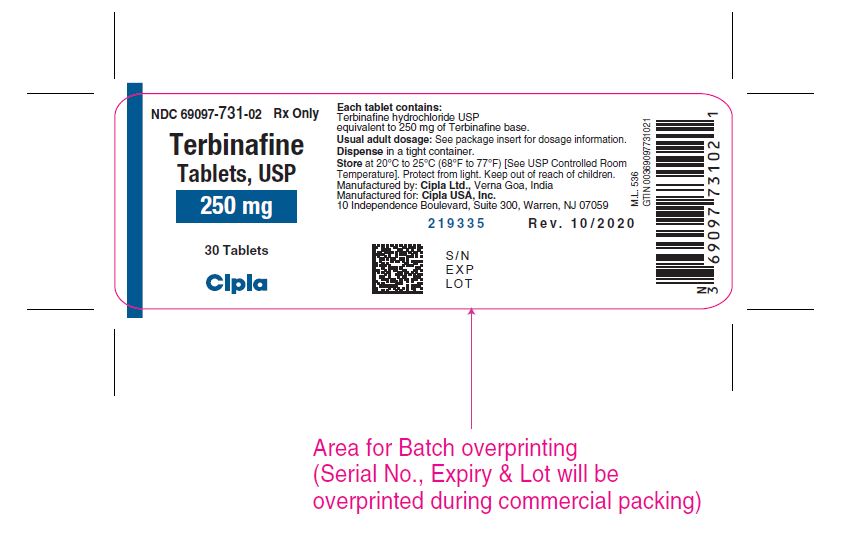
3 DOSAGE FORMS AND STRENGTHSTablet, 250 mg white, circular biconvex tablets debossed with "C134" on one side and plain on the other side.
-
4 CONTRAINDICATIONSTerbinafine tablets are contraindicated in patients with: Chronic or active liver disease [see Warnings and Precautions (5.1)] History of allergic reaction to oral terbinafine because of ...
-
5 WARNINGS AND PRECAUTIONS5.1 Hepatotoxicity - Terbinafine tablets are contraindicated for patients with chronic or active liver disease. Before prescribing terbinafine tablets, perform liver function tests because ...
-
6 ADVERSE REACTIONS6.1 Clinical Trials Experience - Because clinical trials are conducted under widely varying conditions, adverse reaction rates in the clinical trials of a drug cannot be directly compared to ...
-
7 DRUG INTERACTIONS7.1 Drug-Drug Interactions - In vivo studies have shown that terbinafine is an inhibitor of the CYP450 2D6 isozyme. Drugs predominantly metabolized by the CYP450 2D6 isozyme include the ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Available data from postmarketing cases on the use of terbinafine tablets in pregnant women. are insufficient to evaluate a drug-associated risk of major birth ...
-
10 OVERDOSAGEClinical experience regarding overdose with oral terbinafine is limited. Doses up to 5 grams (20 times the therapeutic daily dose) have been taken without inducing serious adverse reactions. The ...
-
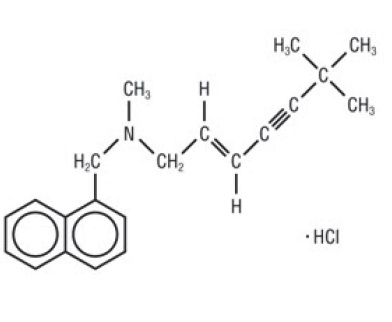
11 DESCRIPTIONTerbinafine tablets, contain the synthetic allylamine antifungal compound terbinafine hydrochloride. Chemically, terbinafine hydrochloride is (E)-N-(6 ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Terbinafine is an allylamine antifungal [see Clinical Pharmacology (12.4)]. 12.2 Pharmacodynamics - The pharmacodynamics of terbinafine tablets is unknown. 12.3 ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - In a 28-month oral carcinogenicity study in rats, an increase in the incidence of liver tumors was observed in males at the highest ...
-
14 CLINICAL STUDIESThe efficacy of terbinafine tablets in the treatment of onychomycosis is illustrated by the response of subjects with toenail and/or fingernail infections who participated in 3 US/Canadian ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGTerbinafine tablets, USP 250 mg are supplied as white, circular biconvex tablets debossed with "C134" on one side and plain on the other side. Bottles of 30 tablets ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-Approved Medication Guide - Patients taking terbinafine tablets should receive the following information and instructions: Advise patients to immediately report ...
-
MEDICATION GUIDEMEDICATION GUIDE - Terbinafine (ter BIN na feen) Tablets, USP - What is the most important information I should know about terbinafine tablets? Terbinafine tablets may cause serious side effects ...
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANELNDC 69097-731-02 Rx only - Terbinafine - Tablets, USP - 250 mg - 30 Tablets - Cipla
-
INGREDIENTS AND APPEARANCEProduct Information