Label: CINACALCET tablet
- NDC Code(s): 69097-410-02, 69097-411-02, 69097-412-02
- Packager: Cipla USA Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated April 29, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use CINACALCET TABLETS safely and effectively. See full prescribing information for CINACALCET TABLETS. CINACALCET tablets, for oral ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE1.1 Secondary Hyperparathyroidism - Cinacalcet tablets are indicated for the treatment of secondary hyperparathyroidism (HPT) in adult patients with chronic kidney disease (CKD) on dialysis [see ...
-
2 DOSAGE AND ADMINISTRATION2.1 Administration - Cinacalcet tablets should be taken with food or shortly after a meal. Cinacalcet tablets are administered orally and should always be taken whole and not chewed, crushed, or ...
-
3 DOSAGE FORMS AND STRENGTHSCinacalcet Tablets 30 mg: Light green colored, oval shaped, biconvex film coated tablets debossed with 'CL' on one side and '410' on other side. Cinacalcet Tablets 60 mg: Light green colored ...
-
4 CONTRAINDICATIONSCinacalcet tablets treatment initiation is contraindicated if serum calcium is less than the lower limit of the normal range [see Warnings and Precautions (5.1)].
-
5 WARNINGS AND PRECAUTIONS5.1 Hypocalcemia - Cinacalcet tablets lowers serum calcium and can lead to hypocalcemia [see Adverse Reactions (6.1)]. Significant lowering of serum calcium can cause paresthesias, myalgias ...
-
6 ADVERSE REACTIONSThe following adverse reactions are discussed in greater detail in other sections of labeling: Hypocalcemia [see Warnings and Precautions (5.1)] Upper Gastrointestinal Bleeding [see ...
-
7 DRUG INTERACTIONS7.1 Strong CYP3A4 Inhibitors - Cinacalcet is partially metabolized by CYP3A4. Dose adjustment of cinacalcet tablets may be required if a patient initiates or discontinues therapy with a strong ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Limited case reports of cinacalcet use in pregnant women are insufficient to inform a drug associated risk of adverse developmental outcomes. In animal reproduction ...
-
10 OVERDOSAGEOverdosage of cinacalcet may lead to hypocalcemia. In the event of overdosage, patients should be monitored for signs and symptoms of hypocalcemia and appropriate measures taken to correct serum ...
-
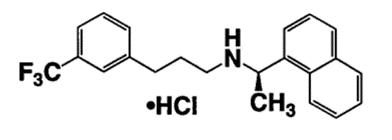
11 DESCRIPTIONCinacalcet tablets contain the hydrochloride salt of cinacalcet, a positive modulator of the calcium sensing receptor. The empirical formula for cinacalcet is C22H22F3N⋅HCl with a molecular ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - The calcium-sensing receptor on the surface of the chief cell of the parathyroid gland is the principal regulator of PTH synthesis and secretion. Cinacalcet, the active ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenicity - Standard lifetime dietary carcinogenicity bioassays were conducted in mice and rats. Mice were given cinacalcet at ...
-
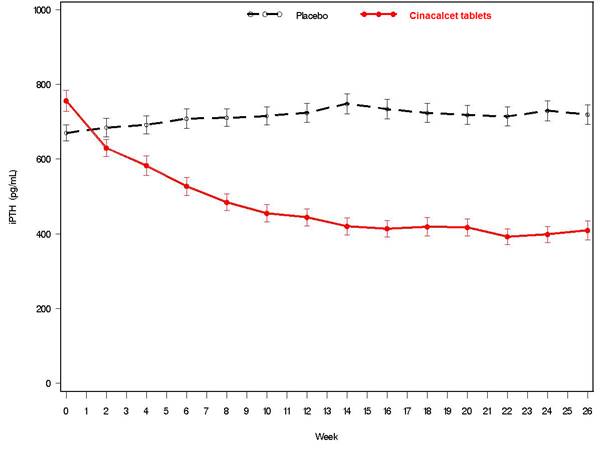
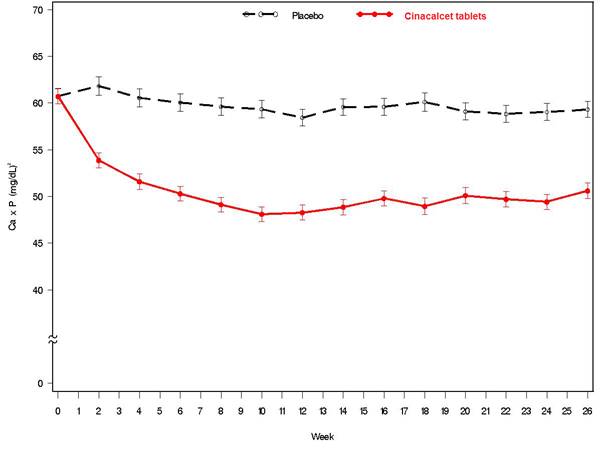
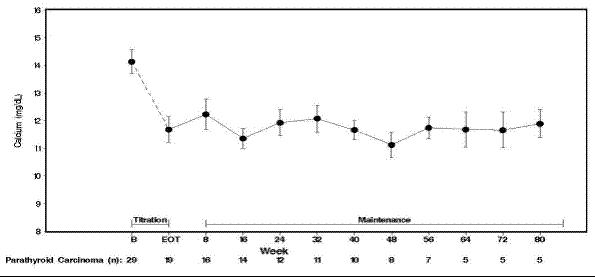
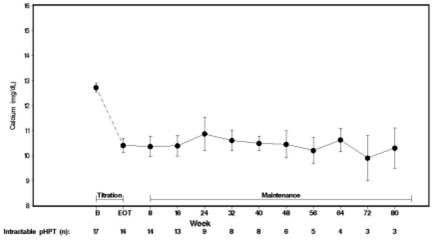
14 CLINICAL STUDIES14.1 Secondary Hyperparathyroidism in Patients with Chronic Kidney Disease on Dialysis - Three 6-month, multicenter, randomized, double-blind, placebo-controlled clinical studies of similar ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGCinacalcet 30 mg tablets are formulated as light-green, film-coated, oval-shaped, biconvex tablets debossed with "CL" on one side and "410" on the opposite side, packaged in bottles of 30 tablets ...
-
17 PATIENT COUNSELING INFORMATIONHypocalcemia: Advise patients to report symptoms of hypocalcemia, including paresthesias, myalgias, muscle spasms, and seizures, to their healthcare provider [see Warnings and Precautions ...
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANELNDC 69097-410-02 Rx Only - Cinacalcet - Tablets - 30mg* 30 Tablets - Cipla - NDC 69097-411-02 Rx Only - Cinacalcet - Tablets - 60mg* 30 Tablets - Cipla - NDC 69097-412-02 Rx ...
-
INGREDIENTS AND APPEARANCEProduct Information