Label: ALBENDAZOLE tablet
- NDC Code(s): 69097-237-72, 69097-237-73
- Packager: Cipla USA Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated December 5, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use ALBENDAZOLE TABLETS safely and effectively. See full prescribing information for ALBENDAZOLE TABLETS. ALBENDAZOLE tablets, for oral ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE1.1 Neurocysticercosis - Albendazole tablets are indicated for the treatment of parenchymal neurocysticercosis due to active lesions caused by larval forms of the pork tapeworm, Taenia ...
-
2 DOSAGE AND ADMINISTRATION2.1 Dosage - Dosing of albendazole tablets will vary depending upon the indication. Albendazole tablets may be crushed or chewed and swallowed with a drink of water. Albendazole tablets should ...
-
3 DOSAGE FORMS AND STRENGTHSTablet: 200 mg
-
4 CONTRAINDICATIONSAlbendazole tablets are contraindicated in patients with known hypersensitivity to the benzimidazole class of compounds or any components of albendazole tablets.
-
5 WARNINGS AND PRECAUTIONS5.1 Bone Marrow Suppression - Fatalities associated with the use of albendazole have been reported due to granulocytopenia or pancytopenia. Albendazole may cause bone marrow suppression ...
-
6 ADVERSE REACTIONS6.1 Clinical Trials Experience - Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly ...
-
7 DRUG INTERACTIONS7.1 Dexamethasone - Steady-state trough concentrations of albendazole sulfoxide were about 56% higher when 8 mg dexamethasone was co-administered with each dose of albendazole (15 mg/kg/day) in ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Based on findings from animal reproduction studies, albendazole may cause fetal harm when administered to a pregnant woman. However, available human data from a ...
-
10 OVERDOSAGEIn case of overdosage, symptomatic therapy and general supportive measures are recommended.
-
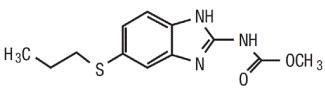
11 DESCRIPTIONAlbendazole tablet, USP is an orally administered anthelmintic drug. Chemically, it is methyl 5-(propylthio)-2-benzimidazolecarbamate. Its molecular formula is C12H15N3O2S. Its molecular weight ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Albendazole is a synthetic, anthelmintic drug of the class benzimidazole [see Clinical Pharmacology (12.4)]. 12.3 Pharmacokinetics - Absorption - Albendazole is ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Long-term carcinogenicity studies were conducted in mice and rats. No evidence of increased incidence of tumors was found in the mice ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING16.1 How Supplied - Albendazole tablet USP, 200 mg are available as white to off-white, circular, bicovex, bevel-edged, film coated tablet debossed with "C237" on one side and plain on other ...
-
17 PATIENT COUNSELING INFORMATIONPatients should be advised that: Some people, particularly children, may experience difficulties swallowing the albendazole tablets whole. Take albendazole tablets with food. Advise ...
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANELRx Only - NDC 69097-237-72 - Albendazole Tablets, USP 200 mg - Each Film coated tablets contain Albendaozle USP, 200 mg - 2 Tablets - Cipla
-
INGREDIENTS AND APPEARANCEProduct Information