Label: GLYCOPYRROLATE tablet
- NDC Code(s): 69076-475-01, 69076-476-01
- Packager: Quinn Pharmaceuticals
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated December 20, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
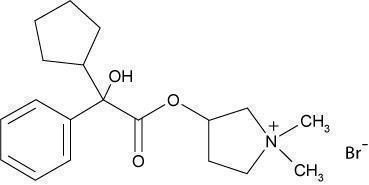
DescriptionGlycopyrrolate tablets contain the synthetic anticholinergic, glycopyrrolate. Glycopyrrolate is a quaternary ammonium compound with the following chemical name ...
-
CLINICAL PHARMACOLOGYGlycopyrrolate, like other anticholinergic (antimuscarinic) agents, inhibits the action of acetylcholine on structures innervated by postganglionic cholinergic nerves and on smooth muscles that ...
-
INDICATIONS AND USAGEFor use as adjunctive therapy in the treatment of peptic ulcer.
-
CONTRAINDICATIONSGlaucoma; obstructive uropathy (for example, bladder neck obstruction due to prostatic hypertrophy); obstructive disease of the gastrointestinal tract (as in achalasia, pyloroduodenal stenosis ...
-
WARNINGSIn the presence of a high environmental temperature, heat prostration (fever and heat stroke due to decreased sweating) can occur with the use of Glycopyrrolate. Diarrhea may be an early symptom ...
-
PRECAUTIONSUse glycopyrrolate with caution in the elderly and in all patients with: Autonomic neuropathy. Hepatic or renal disease. Ulcerative colitis-large doses may suppress intestinal motility to the ...
-
ADVERSE REACTIONSAnticholinergics produce certain effects, most of which are extensions of their fundamental pharmacological actions. Adverse reactions to anticholinergics in general may include xerostomia ...
-
OVERDOSAGEThe symptoms of overdosage of glycopyrrolate are peripheral in nature rather than central. To guard against further absorption of the drug-use gastric avage, cathartics and/or enemas. To combat ...
-
DOSAGE AND ADMINISTRATIONThe dosage of Glycopyrrolate Tablets, USP 1 mg and 2 mg should be adjusted to the needs of the individual patient to assure symptomatic control with a minimum of adverse reactions. The presently ...
-
DRUG INTERACTIONSThere are no known drug interactions.
-
HOW SUPPLIEDGlycopyrrolate Tablets, USP 1 mg are uncoated, round, flat face white tablets with beveled edge and debossed with “0475” on one side and bisected on the other. Glycopyrrolate Tablets, USP 1 mg in ...
-
PRINCIPAL DISPLAY PANELQuinn Pharmaceuticals - NDC 69076-475-01 - Rx only - Glycopyrrolate Tablets, USP - 1 mg - WHITE DYE-FREE ...
-
PRINCIPAL DISPLAY PANELQuinn Pharmaceuticals - NDC 69076-476-01 - Rx only - Glycopyrrolate Tablets, USP - 2 mg - WHITE DYE-FREE ...
-
INGREDIENTS AND APPEARANCEProduct Information