Label: TRETINOIN gel
- NDC Code(s): 68682-800-45
- Packager: Oceanside Pharmaceuticals
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application Authorized Generic
Drug Label Information
Updated February 1, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use TRETINOIN GEL safely and effectively. See full prescribing information for TRETINOIN GEL. TRETINOIN Gel, 0.05%, for topical ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGETretinoin Gel is indicated for topical treatment of acne vulgaris.
-
2 DOSAGE AND ADMINISTRATIONFor topical use only. Not for oral, ophthalmic, or intravaginal use. Tretinoin Gel should be applied once daily, before bedtime, to the skin where acne lesions appear, using a thin layer to cover ...
-
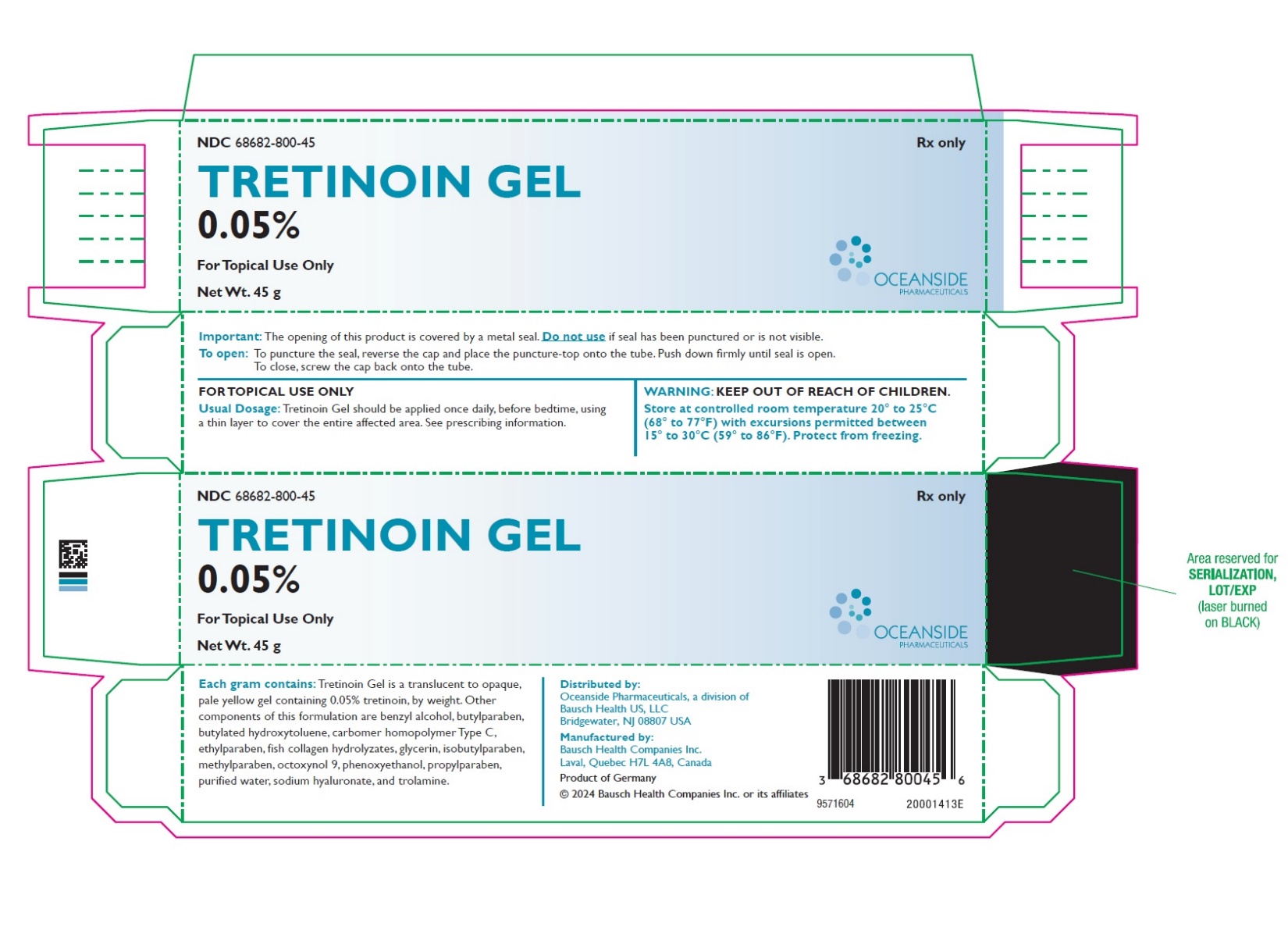
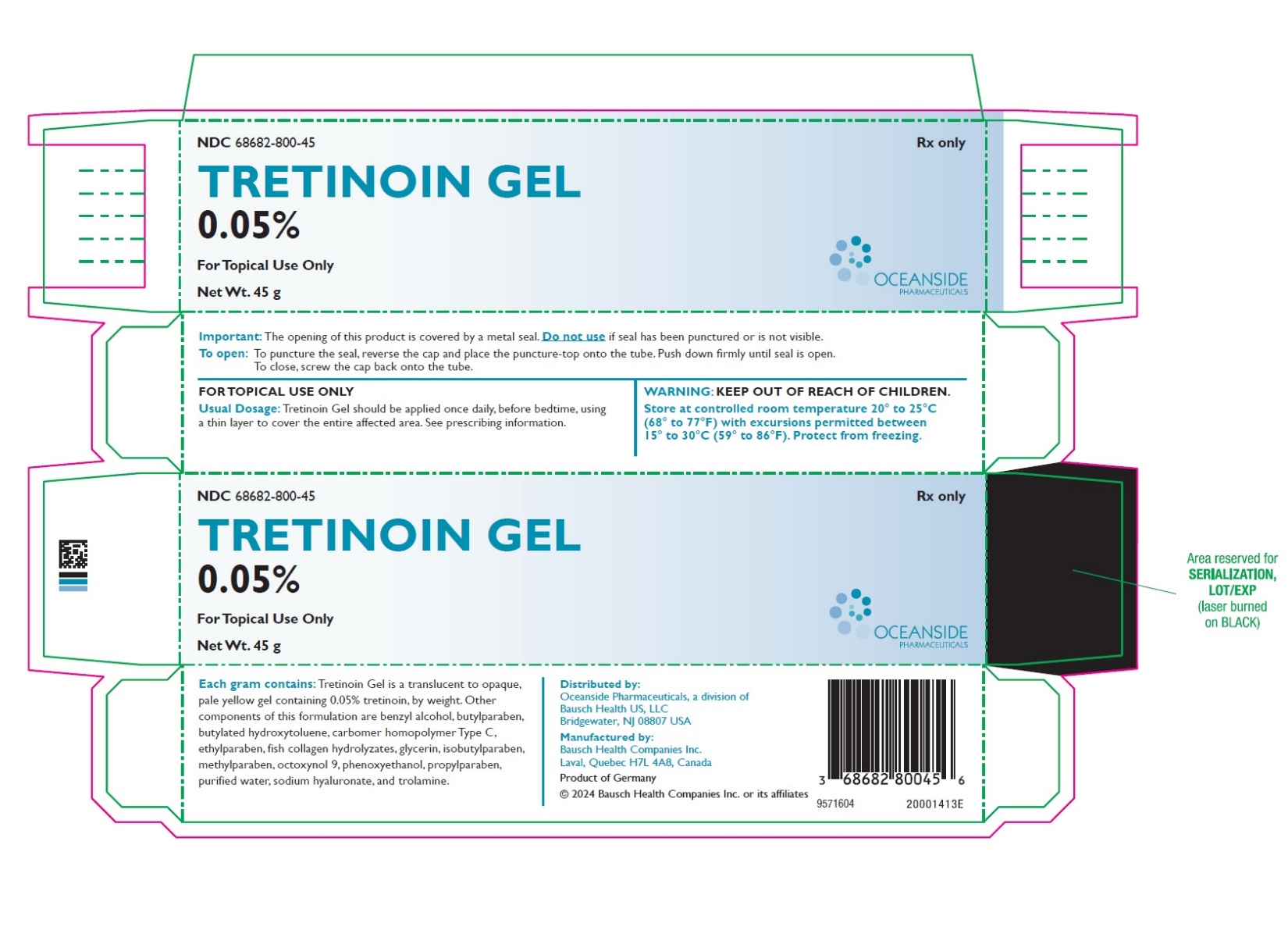
3 DOSAGE FORMS AND STRENGTHSGel, 0.05% Each gram of Tretinoin Gel contains 0.5 mg (0.05%) tretinoin in a translucent to opaque, pale yellow topical gel.
-
4 CONTRAINDICATIONSNone.
-
5 WARNINGS AND PRECAUTIONS5.1 Skin Irritation - The skin of certain individuals may become dry, red, or exfoliated while using Tretinoin Gel. If the degree of irritation warrants, patients should be directed to ...
-
6 ADVERSE REACTIONS6.1 Clinical Trials Experience - Because clinical trials are conducted under prescribing conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - There are no well-controlled trials in pregnant women treated with Tretinoin Gel. Tretinoin Gel should be used during pregnancy only if the potential benefit justifies the ...
-
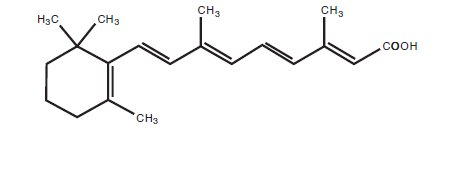
11 DESCRIPTIONTretinoin Gel, 0.05% is a translucent to opaque, pale yellow gel containing 0.05% tretinoin, by weight for topical administration. Chemically, tretinoin is all-trans-retinoic acid, also known as ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Tretinoin is a metabolite of Vitamin A that binds with high affinity to specific retinoic acid receptors located in both the cytosol and nucleus, but cutaneous levels ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - A 2-year dermal mouse carcinogenicity study was initiated with topical administration of 0.005%, 0.025% and 0.05% Tretinoin Gel ...
-
14 CLINICAL STUDIESThe safety and efficacy of Tretinoin Gel used once daily before bedtime for the treatment of mild to moderate acne vulgaris were assessed in two 12-week prospective, multicenter, randomized ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGTretinoin Gel, 0.05% is a translucent to opaque, pale yellow topical gel and available as: • NDC 68682-800-45 45 g tube - Storage and Handling: Store at controlled room temperature 20° to ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Patient Information). Instruct patients to clean the affected areas with an appropriate cleanser before applying Tretinoin ...
-
PATIENT PACKAGE INSERTPatient Information - Tretinoin Gel, 0.05% for topical use - Important information: Tretinoin Gel is for use on skin only. Do not get Tretinoin Gel in your mouth, eyes, vagina, or the corners of ...
-
PRINCIPAL DISPLAY PANELPRINCIPAL DISPLAY PANEL - 45 g Tube Carton - NDC 68682-800-45 - Rx only - TRETINOIN GEL - 0.05% FOR TOPICAL USE ONLY - Net Wt. 45 g - OCEANSIDE - PHARMACEUTICALS
-
INGREDIENTS AND APPEARANCEProduct Information