Label: CLINDAMYCIN PHOSPHATE 1.2% AND TRETINOIN 0.025%- clindamycin phosphate and tretinoin gel
- NDC Code(s): 68682-300-30, 68682-300-60
- Packager: Oceanside Pharmaceuticals
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application Authorized Generic
Drug Label Information
Updated April 1, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use CLINDAMYCIN PHOSPHATE 1.2% and TRETINOIN 0.025% Gel safely and effectively. See full prescribing information for CLINDAMYCIN ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEClindamycin Phosphate 1.2% and Tretinoin 0.025% Gel is indicated for the topical treatment of acne vulgaris in patients 12 years or older.
-
2 DOSAGE AND ADMINISTRATIONAt bedtime, squeeze a pea-sized amount of medication onto one fingertip, dot onto the chin, cheeks, nose, and forehead, then gently rub over the entire face. Clindamycin Phosphate 1.2% and ...
-
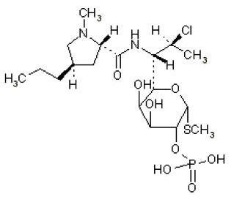
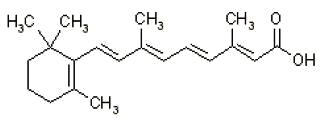
3 DOSAGE FORMS AND STRENGTHSClindamycin Phosphate 1.2% and Tretinoin 0.025% Gel, a combination of a lincosamide antibiotic and a retinoid, contains clindamycin phosphate 1.2% and tretinoin 0.025%, formulated as a topical ...
-
4 CONTRAINDICATIONSClindamycin Phosphate 1.2% and Tretinoin 0.025% Gel is contraindicated in patients with regional enteritis, ulcerative colitis, or history of antibiotic-associated colitis.
-
5 WARNINGS AND PRECAUTIONS5.1 Colitis - Systemic absorption of clindamycin has been demonstrated following topical use of this product. Diarrhea, bloody diarrhea, and colitis (including pseudomembranous colitis) have been ...
-
6 ADVERSE REACTIONS6.1 Clinical Studies Experience - Because clinical trials are conducted under prescribed conditions, adverse reaction rates observed in the clinical trial may not reflect the rates observed in ...
-
7 DRUG INTERACTIONS7.1 Concomitant Topical Medication - Concomitant topical medication, medicated or abrasive soaps and cleansers, soaps and cosmetics that have a strong drying effect, and products with high ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Pregnancy Category C. There are no well-controlled trials in pregnant women treated with Clindamycin Phosphate 1.2% and Tretinoin 0.025% Gel. Clindamycin Phosphate 1.2% and ...
-
11 DESCRIPTIONClindamycin Phosphate 1.2% and Tretinoin 0.025% Gel is an antibiotic and retinoid combination gel product with two active ingredients. Clindamycin phosphate is a water-soluble ester of the ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Clindamycin - [See Microbiology (12.4).] Tretinoin - Although the exact mode of action of tretinoin is unknown, current evidence suggests that topical ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenicity, mutagenicity and impairment of fertility testing of Clindamycin Phosphate 1.2% and Tretinoin 0.025% Gel have not been ...
-
14 CLINICAL STUDIESThe safety and efficacy of once daily use of Clindamycin Phosphate 1.2% and Tretinoin 0.025% Gel for treatment of acne vulgaris were assessed in three 12-week prospective, multi-center ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGClindamycin Phosphate 1.2% and Tretinoin 0.025% Gel is supplied as follows: 30 gram tube NDC 68682-300-30 - 60 gram tube NDC 68682-300-60 ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Patient Information). Instructions for Use - • At bedtime, the face should be gently washed with a mild soap and warm water. After ...
-
PATIENT PACKAGE INSERTPATIENT INFORMATION - Clindamycin Phosphate 1.2% and Tretinoin 0.025% Gel - IMPORTANT: Not for mouth, eye, or vaginal use. Read the Patient Information that comes with Clindamycin Phosphate 1.2 ...
-
PRINCIPAL DISPLAY PANELPRINCIPAL DISPLAY PANEL - 60 g Carton - NDC 68682-300-60 - Rx only - CLINDAMYCIN PHOSPHATE 1.2% and TRETINOIN 0.025% GEL - For Topical Use Only - Not For Ophthalmic, Oral, Or Intravaginal ...
-
INGREDIENTS AND APPEARANCEProduct Information