Label: BENAZEPRIL HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE tablet, film coated
- NDC Code(s): 68462-576-01, 68462-577-01, 68462-578-01, 68462-579-01
- Packager: Glenmark Pharmaceuticals Inc., USA
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated September 30, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
BOXED WARNING
(What is this?)
WARNING: FETAL TOXICITY
When pregnancy is detected, discontinue benazepril hydrochloride and hydrochlorothiazide as soon as possible.
Drugs that act directly on the renin-angiotensin system can cause injury and death to the developing fetus (see WARNINGS, Fetal Toxicity).
Close -
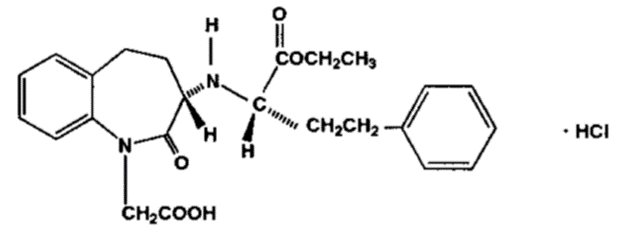
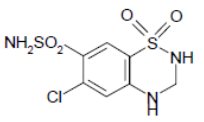
DESCRIPTIONBenazepril hydrochloride, USP is a white to off-white crystalline powder, soluble (>100 mg/mL) in water, in ethanol, and in methanol. Benazepril hydrochloride's chemical name is ...
-
CLINICAL PHARMACOLOGYMechanism of Action - Benazepril and benazeprilat inhibit angiotensin-converting enzyme (ACE) in human subjects and in animals. ACE is a peptidyl dipeptidase that catalyzes the conversion of ...
-
CLINICAL STUDIESIn single-dose studies, benazepril lowered blood pressure within 1 hour, with peak reductions achieved 2 to 4 hours after dosing. The antihypertensive effect of a single dose persisted for 24 ...
-
INDICATIONS AND USAGEBenazepril hydrochloride and hydrochlorothiazide tablets are indicated for the treatment of hypertension. This fixed combination drug is not indicated for the initial therapy of hypertension (see ...
-
CONTRAINDICATIONSBenazepril hydrochloride and hydrochlorothiazide is contraindicated in patients who are anuric. Benazepril hydrochloride and hydrochlorothiazide is also contraindicated in patients who are ...
-
WARNINGSAnaphylactoid and Possibly Related Reactions - Presumably because angiotensin-converting enzyme inhibitors affect the metabolism of eicosanoids and polypeptides, including endogenous bradykinin ...
-
PRECAUTIONSGeneral - Serum Electrolyte Abnormalities - In clinical trials of benazepril hydrochloride and hydrochlorothiazide, the average change in serum potassium was near zero in subjects who ...
-
ADVERSE REACTIONSBenazepril hydrochloride and hydrochlorothiazide has been evaluated for safety in over 2500 patients with hypertension; over 500 of these patients were treated for at least 6 months, and over 200 ...
-
OVERDOSAGENo specific information is available on the treatment of overdosage with benazepril hydrochloride and hydrochlorothiazide; treatment should be symptomatic and supportive. Therapy with benazepril ...
-
DOSAGE AND ADMINISTRATIONDose once daily. The dosage may then be increased after 2 to 3 weeks as needed to help achieve blood pressure goals. The maximum recommended dose is 20/25 mg. Switch Therapy - A patient whose ...
-
HOW SUPPLIEDBenazepril Hydrochloride and Hydrochlorothiazide Tablets, USP for oral administration, are available as - 5 mg/6.25 mg - White to off-white, oblong, film-coated tablets, debossed "E 124" on one ...
-
Package/Label Display PanelNDC 68462-576-01 - Benazepril - Hydrochloride and - Hydrochlorothiazide - Tablets, USP - 5 mg/6.25 mg - Rx only - 100 Tablets
-
Package/Label Display PanelNDC 68462-577-01 - Benazepril - Hydrochloride and - Hydrochlorothiazide - Tablets, USP - 10 mg/12.5 mg - Rx only - 100 Tablets
-
Package/Label Display PanelNDC 68462-578-01 - Benazepril - Hydrochloride and - Hydrochlorothiazide - Tablets, USP - 20 mg/12.5 mg - Rx only - 100 Tablets
-
Package/Label Display PanelNDC 68462-579-01 - Benazepril - Hydrochloride and - Hydrochlorothiazide - Tablets, USP - 20 mg/25 mg - Rx only - 100 Tablets
-
INGREDIENTS AND APPEARANCEProduct Information