Label: CLINDAMYCIN AND BENZOYL PEROXIDE gel
- NDC Code(s): 68462-486-19, 68462-486-24, 68462-486-27, 68462-859-24, view more
- Packager: Glenmark Pharmaceuticals Inc., USA
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated August 31, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
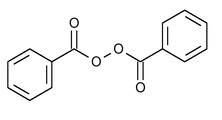
SPL UNCLASSIFIED SECTIONTopical Gel: clindamycin (1%) as clindamycin phosphate, benzoyl peroxide (5%) For Dermatological Use Only - Not for Ophthalmic Use - *Reconstitute Before Dispensing*
-
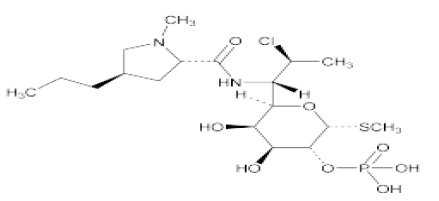
DESCRIPTIONClindamycin and Benzoyl Peroxide Gel, 1%/5% contains clindamycin phosphate, (7(S)-chloro-7-deoxylincomycin-2-phosphate). Clindamycin phosphate is a water soluble ester of the semi-synthetic ...
-
CLINICAL PHARMACOLOGYAn in vitro percutaneous penetration study comparing Clindamycin and Benzoyl Peroxide Gel and topical 1% clindamycin gel alone, demonstrated there was no statistical difference in penetration ...
-
CLINICAL STUDIESIn two adequate and well controlled clinical studies of 758 patients, 214 used clindamycin and benzoyl peroxide gel, 210 used benzoyl peroxide, 168 used clindamycin, and 166 used vehicle ...
-
INDICATIONS AND USAGEClindamycin and Benzoyl Peroxide Gel is indicated for the topical treatment of acne vulgaris.
-
CONTRAINDICATIONSClindamycin and Benzoyl Peroxide Gel is contraindicated in those individuals who have shown hypersensitivity to any of its components or to lincomycin. It is also contraindicated in those having ...
-
WARNINGSORALLY AND PARENTERALLY ADMINISTERED CLINDAMYCIN HAS BEEN ASSOCIATED WITH SEVERE COLITIS WHICH MAY RESULT IN PATIENT DEATH. USE OF THE TOPICAL FORMULATION OF CLINDAMYCIN RESULTS IN ABSORPTION OF ...
-
PRECAUTIONSGeneral: For dermatological use only; not for ophthalmic use. Concomitant topical acne therapy should be used with caution because a possible cumulative irritancy effect may occur, especially ...
-
ADVERSE REACTIONSDuring clinical trials, the most frequently reported adverse event in the clindamycin and benzoyl peroxide gel treatment group was dry skin (12%). The Table below lists local adverse events ...
-
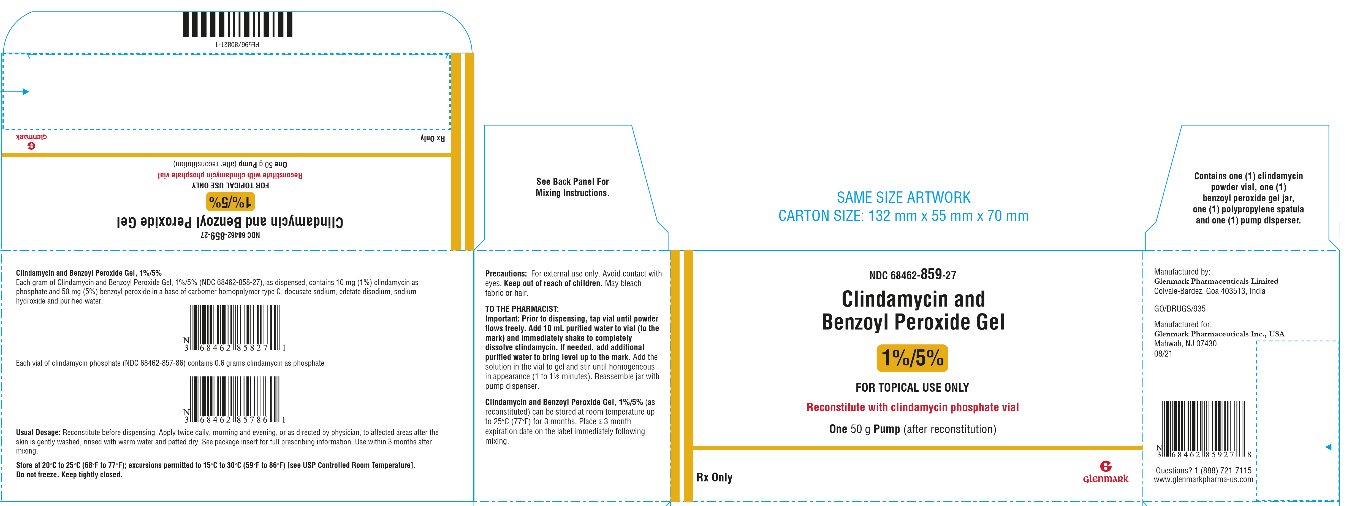
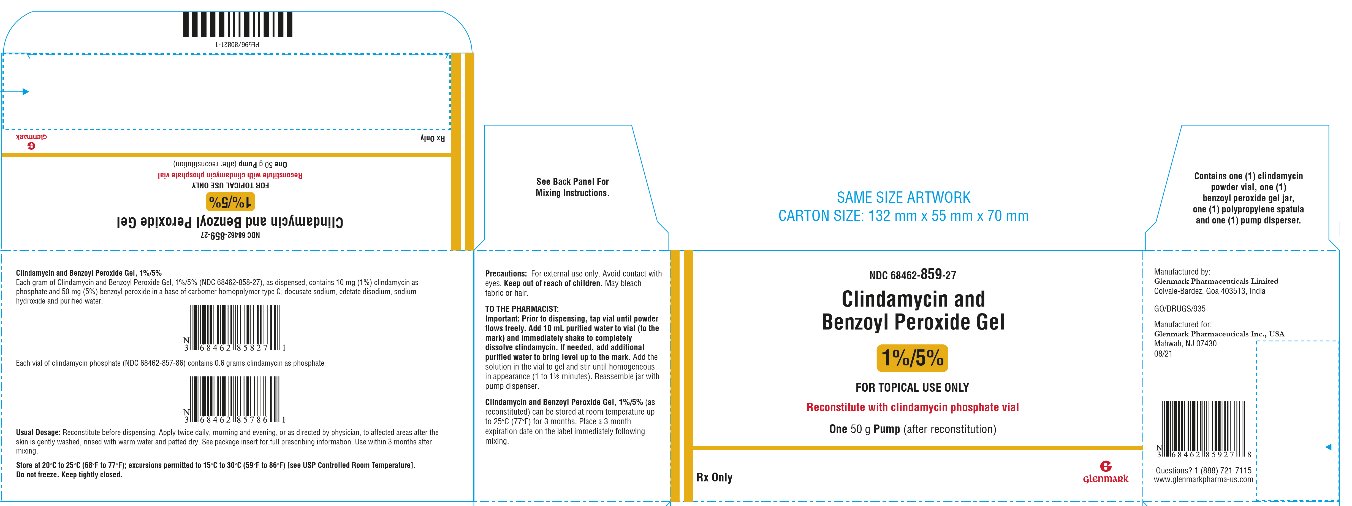
DOSAGE AND ADMINISTRATIONClindamycin and Benzoyl Peroxide Gel should be applied twice daily, morning and evening, or as directed by a physician, to affected areas after the skin is gently washed, rinsed with warm water ...
-
HOW SUPPLIED AND COMPOUNDING INSTRUCTIONSSize - (Net Weight) NDC Number - Benzoyl Peroxide Gel - Active Clindamycin powder (In plastic vial) Purified Water To Be Added to each vial - 25 grams - 68462-486-19 - 19.7g - 0.3g - 5 ...
-
STORAGE AND HANDLINGStore at 20°C to 25°C (68°F to 77°F); excursions permitted to 15°C to 30°C (59°F to 86°F) [see USP Controlled Room Temperature]. Do not freeze. Keep tightly closed. Keep out of reach of ...
-
Label Display Panel – 25 gram Kit Carton

-
Label Display Panel - 35 gram Kit Carton

-
Label Display Panel – 50 gram Kit Carton

-
PRINCIPAL DISPLAY PANEL – 35 g Pump Pack

-
PRINCIPAL DISPLAY PANEL – 50 g Pump Pack
-
INGREDIENTS AND APPEARANCEProduct Information