Label: TROSPIUM CHLORIDE tablet, film coated
- NDC Code(s): 68462-461-05, 68462-461-10, 68462-461-30, 68462-461-60
- Packager: Glenmark Pharmaceuticals Inc., USA
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated December 28, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use TROSPIUM CHLORIDE TABLETS safely and effectively. See full prescribing information for TROSPIUM CHLORIDE TABLETS. TROSPIUM ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGETrospium chloride tablets are a muscarinic antagonist indicated for the treatment of overactive bladder (OAB) with symptoms of urge urinary incontinence, urgency, and urinary frequency.
-
2 DOSAGE AND ADMINISTRATIONThe recommended dose is 20 mg twice daily. Trospium chloride tablets should be dosed at least one hour before meals or given on an empty stomach. Dosage modification is recommended in the ...
-
3 DOSAGE FORMS AND STRENGTHSTrospium Chloride Tablets, USP are supplied as 20 mg tablets (brownish yellow, round, biconvex film-coated tablets, debossed with ‘L’ on one side and ‘1’ on the other side).
-
4 CONTRAINDICATIONSTrospium chloride is contraindicated in patients with: • urinary retention - • gastric retention - • uncontrolled narrow-angle glaucoma. • known hypersensitivity to the drug or its ingredients ...
-
5 WARNINGS AND PRECAUTIONS5.1 Risk of Urinary Retention - Trospium chloride should be administered with caution to patients with clinically significant bladder outflow obstruction because of the risk of urinary retention ...
-
6 ADVERSE REACTIONS6.1 Clinical Trials Experience - Because clinical trials are conducted under widely varying conditions, the adverse reaction rates observed in the clinical trials of a drug cannot be directly ...
-
7 DRUG INTERACTIONS7.1 Digoxin - Concomitant use of trospium chloride and digoxin did not affect the pharmacokinetics of either drug [see Clinical Pharmacology (12.3)]. 7.2 Drugs Eliminated by Active Tubular ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Teratogenic Effects - There are no adequate and well-controlled studies of trospium chloride in pregnant women. Trospium chloride should be used during pregnancy only if the ...
-
10 OverdosageOverdosage with antimuscarinic agents, including trospium chloride, can result in severe antimuscarinic effects. Supportive treatment should be provided according to symptoms. In the event of ...
-
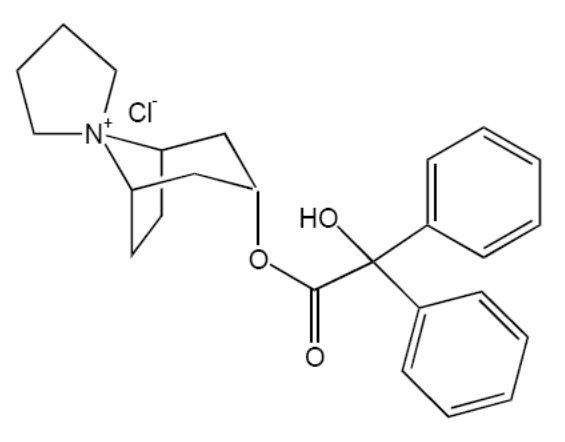
11 DESCRIPTIONTrospium chloride, USP is a quaternary ammonium compound with the chemical name of Spiro [8-azoniabicyclo[3.2.1]octane-8,1'-pyrrolidinium], 3-[(hydroxydiphenylacetyl)oxy]-, chloride, (1R, 3r, 5S) ...
-
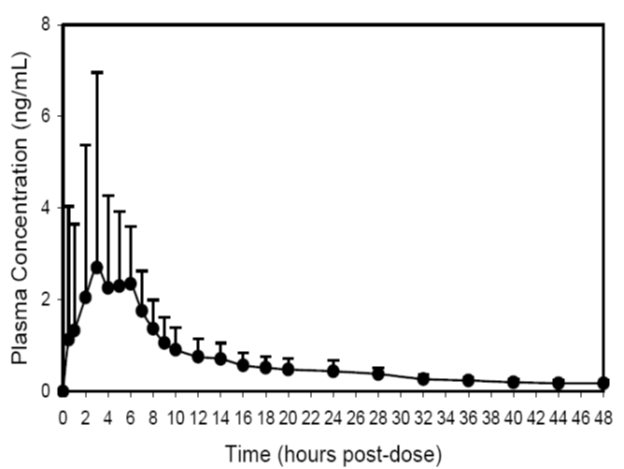
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Trospium chloride is a muscarinic antagonist. Trospium chloride antagonizes the effect of acetylcholine on muscarinic receptors in cholinergically innervated organs ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis: Carcinogenicity studies with trospium chloride were conducted in mice and rats for 78 weeks and 104 weeks ...
-
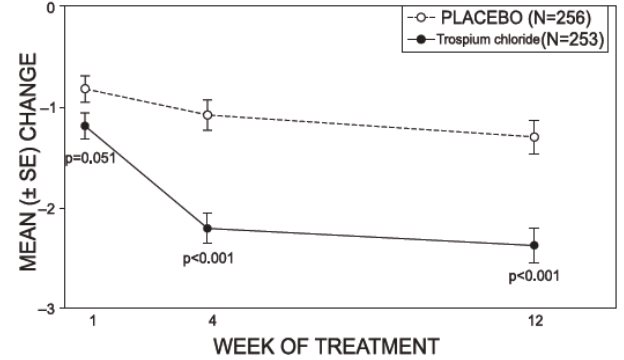
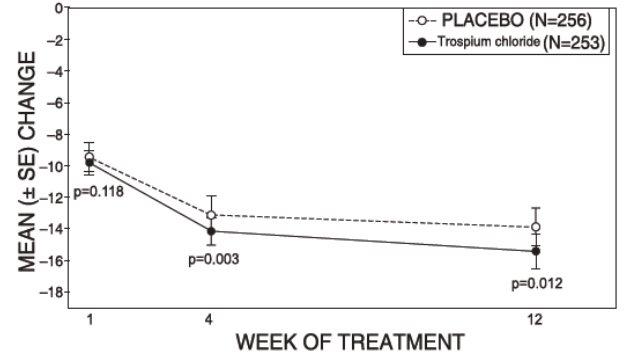
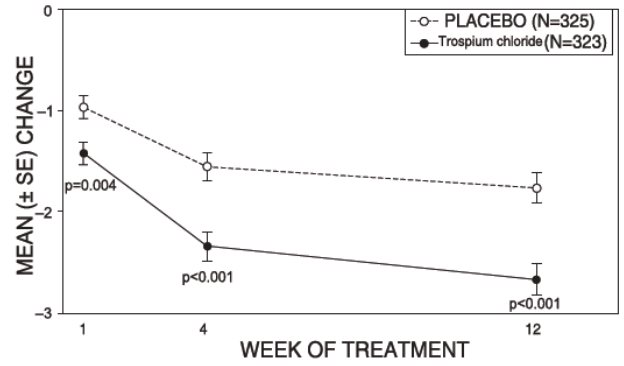
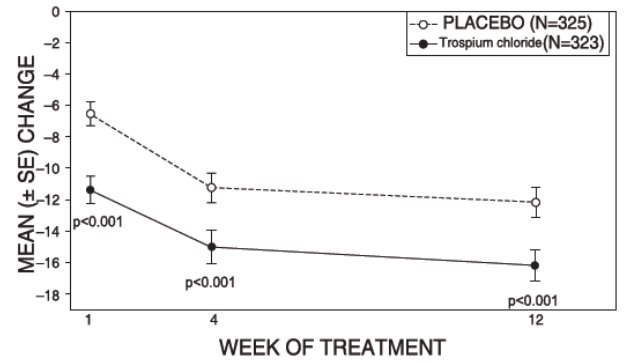
14 CLINICAL STUDIESTrospium chloride was evaluated for the treatment of patients with overactive bladder who had symptoms of urinary frequency, urgency, and urge incontinence in two U.S. 12-week, placebo-controlled ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGTrospium Chloride Tablets, USP, 20 mg (brownish yellow, round, biconvex film-coated tablets, debossed with ‘L’ on one side and ‘1’ on the other side) are supplied as follows: Bottles of 30 NDC ...
-
17 PATIENT COUNSELING INFORMATION“See FDA-approved Patient Labeling (Patient Information)” 17.1 Angioedema - Patients should be informed that trospium chloride, the active ingredient in trospium chloride tablets, may produce ...
-
PATIENT INFORMATIONTrospium Chloride (trose’ pee um klor’ ide) Tablets - Read the Patient Information that comes with trospium chloride tablets before you start taking it and each time you get a refill. There may ...
-
PACKAGE/LABEL PRINCIPAL DISPLAY PANELNDC 68462-461-60 - TROSPIUM CHLORIDE TABLETS USP - 20 mg - Pharmacist: Dispense the patient information sheet provided separately to each patient.
-
INGREDIENTS AND APPEARANCEProduct Information