Label: SOLIFENACIN SUCCINATE tablet, film coated
SOLIFENACIN SUCCINATE tablet, film coated, extended release
- NDC Code(s): 68462-386-14, 68462-386-30, 68462-386-90, 68462-387-14, view more
- Packager: Glenmark Pharmaceuticals Inc., USA
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated July 30, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use SOLIFENACIN SUCCINATE TABLETS safely and effectively. See full prescribing information for SOLIFENACIN SUCCINATE ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGESolifenacin succinate tablets are indicated for the treatment of adults with overactive bladder with symptoms of urge urinary incontinence, urgency, and urinary frequency.
-
2 DOSAGE AND ADMINISTRATION2.1 Dosing Information - The recommended oral dose of solifenacin succinate tablets is 5 mg once daily. If the 5 mg dose is well tolerated, the dose may be increased to 10 mg once ...
-
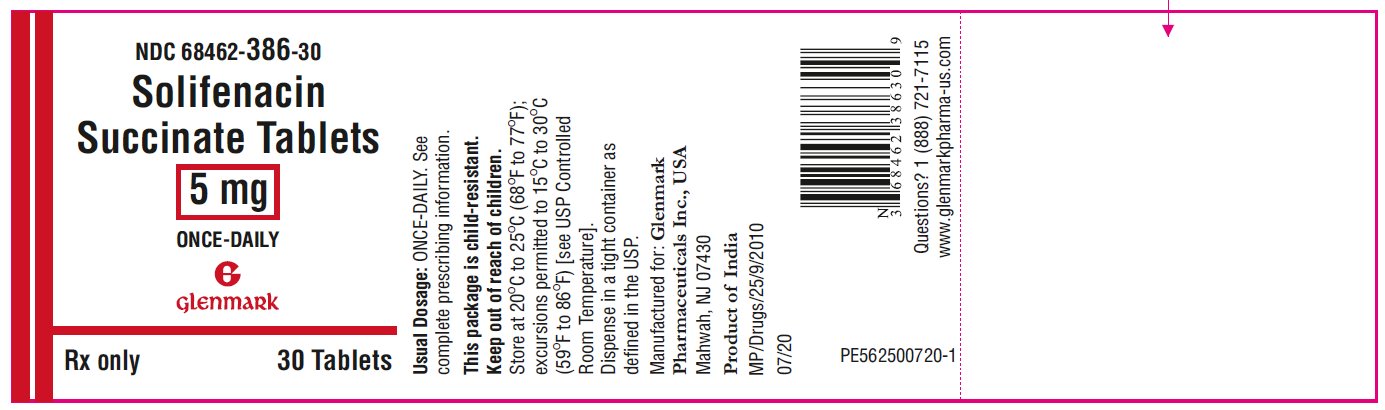
3 DOSAGE FORMS AND STRENGTHSThe 5 mg tablets are yellow colored, round, biconvex, film-coated tablets with ‘G’ debossed on one side and ‘51’ debossed on the other side. The 10 mg tablets are pink colored, round, biconvex ...
-
4 CONTRAINDICATIONSSolifenacin succinate tablets are contraindicated in patients: • With urinary retention [see Warnings and Precautions (5.2)], • With gastric retention [see Warnings and Precautions (5.3)], • With ...
-
5 WARNINGS AND PRECAUTIONS5.1 Angioedema and Anaphylactic Reactions - Angioedema of the face, lips, tongue, and/or larynx have been reported with solifenacin succinate. In some cases, angioedema occurred after the first ...
-
6 ADVERSE REACTIONS6.1 Clinical Trials Experience - Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly ...
-
7 DRUG INTERACTIONS7.1 Strong CYP3A4 Inhibitors - Solifenacin is a substrate of CYP3A4. Concomitant use of ketoconazole, a strong CYP3A4 inhibitor, significantly increased the exposure of solifenacin [see Clinical ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - There are no studies with the use of solifenacin succinate in pregnant women to inform a drug-associated risk of major birth defects, miscarriages, or adverse ...
-
10 OVERDOSAGEOverdosage with solifenacin succinate can potentially result in severe antimuscarinic effects and should be treated accordingly. The highest dose ingested in an accidental overdose of solifenacin ...
-
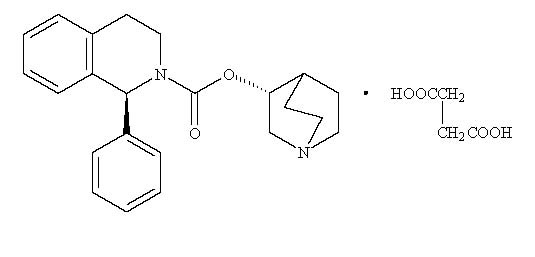
11 DESCRIPTIONSolifenacin succinate tablets are a muscarinic receptor antagonist. Chemically, solifenacin succinate is a butanedioic acid compound with 1(S)-phenyl-1,2,3,4-tetrahydroisoquinoline-2-carboxylic ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Solifenacin is a competitive muscarinic receptor antagonist. Muscarinic receptors play an important role in several major cholinergically mediated functions, including ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - No increase in tumors was found following the administration of solifenacin succinate to male and female mice for 104 weeks at doses up ...
-
14 CLINICAL STUDIESSolifenacin succinate was evaluated in four twelve-week, double-blind, randomized, placebo-controlled, parallel group, multicenter clinical trials for the treatment of overactive bladder in adult ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGSolifenacin Succinate Tablets are supplied as round, biconvex, film-coated tablets, available in bottles and unit-dose blister packages as follows: Each 5 mg tablet is yellow colored and ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Patient Information). Angioedema and Anaphylactic Reactions - Inform patients that angioedema and anaphylactic reactions have been ...
-
Patient InformationSolifenacin Succinate - (SOE-li-FEN-a-sin SUX-i-nate) Tablets - Read the Patient Information that comes with solifenacin succinate tablets before you start taking it and each time you get a ...
-
Package/Label Display Panel NDC 68462-386-30 - Solifenacin Succinate Tablets, 5 mg - 30 Tablets-Bottle
-
Package/Label Display Panel NDC 68462-387-30 - Solifenacin Succinate Tablets, 10 mg - 30 Tablets-Bottle
-
INGREDIENTS AND APPEARANCEProduct Information