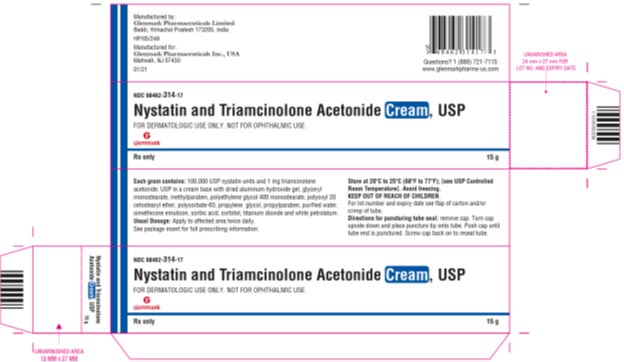
Label: NYSTATIN AND TRIAMCINOLONE ACETONIDE cream
- NDC Code(s): 68462-314-17, 68462-314-35, 68462-314-65
- Packager: Glenmark Pharmaceuticals Inc., USA
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated January 31, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONFOR EXTERNAL USE ONLY. NOT FOR OPHTHALMIC USE.
-
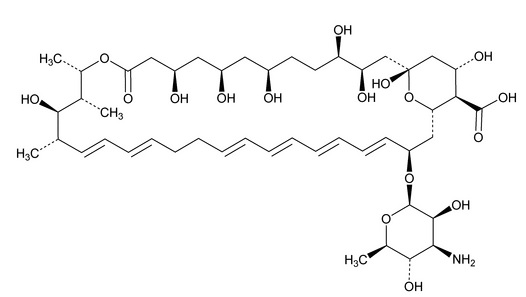
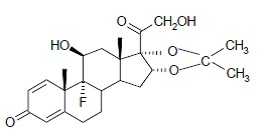
DESCRIPTIONNystatin and Triamcinolone Acetonide Cream, USP for dermatologic use contains the antifungal agent nystatin, USP and the synthetic corticosteroid triamcinolone acetonide, USP. Nystatin, USP is a ...
-
CLINICAL PHARMACOLOGYNystatin - Nystatin exerts its antifungal activity against a variety of pathogenic and nonpathogenic yeasts and fungi by binding to sterols in the cell membrane. The binding process renders the ...
-
INDICATIONS AND USAGENystatin and triamcinolone acetonide cream is indicated for the treatment of cutaneous candidiasis; it has been demonstrated that the nystatin-steroid combination provides greater benefit than the ...
-
CONTRAINDICATIONSThese preparations are contraindicated in those patients with a history of hypersensitivity to any of their components.
-
PRECAUTIONSGeneral - Systemic absorption of topical corticosteroids has produced reversible hypothalamic-pituitary-adrenal (HPA) axis suppression, manifestations of Cushing's syndrome, hyperglycemia, and ...
-
ADVERSE REACTIONSA single case (approximately one percent of patients studied) of acneiform eruption occurred with use of combined nystatin and triamcinolone acetonide in clinical studies. Nystatin is virtually ...
-
OVERDOSAGETopically applied corticosteroids can be absorbed in sufficient amounts to produce systemic effects (see PRECAUTIONS, General); however, acute overdosage and serious adverse effects with ...
-
DOSAGE AND ADMINISTRATIONNystatin and triamcinolone acetonide cream is usually applied to the affected areas twice daily in the morning and evening by gently and thoroughly massaging the preparation into the skin. The ...
-
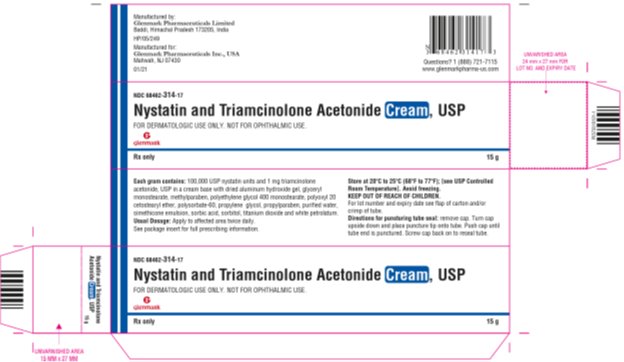
HOW SUPPLIEDNystatin and Triamcinolone Acetonide Cream, USP is supplied as follows: NDC 68462-314-17 15g tube (1 tube per carton) NDC 68462-314-35 30g tube (1 tube per carton) NDC 68462-314-65 60g ...
-
SPL UNCLASSIFIED SECTIONManufactured by: Glenmark Pharmaceuticals Limited - Baddi, Himachal Pradesh 173205, India - Manufactured for: Glenmark Pharmaceuticals Inc., USA - Mahwah, NJ 07430 - Questions? 1 (888 ...
-
PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCEProduct Information