Label: BRIMONIDINE TARTRATE/TIMOLOL MALEATE- brimonidine tartrate and timolol maleate solution/ drops
- NDC Code(s): 68462-281-32, 68462-281-69
- Packager: Glenmark Pharmaceuticals Inc., USA
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated May 16, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use BRIMONIDINE TARTRATE/TIMOLOL MALEATE OPHTHALMIC SOLUTION safely and effectively. See full prescribing information for BRIMONIDINE ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEBrimonidine tartrate/timolol maleate ophthalmic solution 0.2%/0.5% is an alpha-adrenergic receptor agonist with a beta-adrenergic receptor inhibitor indicated for the reduction of elevated ...
-
2 DOSAGE AND ADMINISTRATIONThe recommended dose is one drop of brimonidine tartrate/timolol maleate ophthalmic solution in the affected eye(s) twice daily approximately 12 hours apart. If more than one topical ophthalmic ...
-
3 DOSAGE FORMS AND STRENGTHSSolution containing 2 mg/mL brimonidine tartrate and 5 mg/mL timolol (6.8 mg/mL timolol maleate).
-
4 CONTRAINDICATIONS4.1 Reactive Airway Disease Including Asthma, COPD - Brimonidine tartrate/timolol maleate ophthalmic solution is contraindicated in patients with reactive airway disease including bronchial ...
-
5 WARNINGS AND PRECAUTIONS5.1 Potential for Severe Respiratory or Cardiac Reactions - Brimonidine tartrate/timolol maleate ophthalmic solution contains timolol maleate; and although administered topically can be absorbed ...
-
6 ADVERSE REACTIONS6.1 Clinical Studies Experience - Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a drug cannot be directly ...
-
7 DRUG INTERACTIONS7.1 Antihypertensives/Cardiac Glycosides - Because brimonidine tartrate/timolol maleate ophthalmic solution may reduce blood pressure, caution in using drugs such as antihypertensives and/or ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Teratogenicity studies have been performed in animals. Brimonidine tartrate was not teratogenic when given orally during gestation days 6 through 15 in rats and days 6 through 18 ...
-
10 OVERDOSAGEThere have been reports of inadvertent overdosage with timolol ophthalmic solution resulting in systemic effects similar to those seen with systemic beta-adrenergic blocking agents such as ...
-
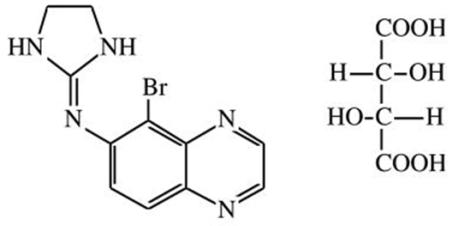
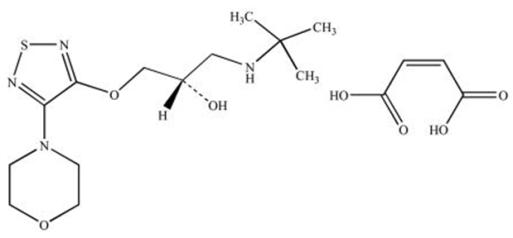
11 DESCRIPTIONBrimonidine Tartrate/Timolol Maleate Ophthalmic Solution 0.2%/0.5%, sterile, is a relatively selective alpha-2 adrenergic receptor agonist with a non-selective beta-adrenergic receptor inhibitor ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Brimonidine tartrate/timolol maleate ophthalmic solution is comprised of two components: brimonidine tartrate and timolol. Each of these two components decreases ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - With brimonidine tartrate, no compound-related carcinogenic effects were observed in either mice or rats following a 21-month and ...
-
14 CLINICAL STUDIESClinical studies were conducted to compare the IOP-lowering effect over the course of the day of brimonidine tartrate/timolol maleate ophthalmic solution administered twice a day (BID) to ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING Brimonidine Tartrate/Timolol Maleate Ophthalmic Solution is a clear slightly greenish-yellow solution and is supplied sterile, in white opaque plastic LDPE bottles and dropper, with blue high ...
-
17 PATIENT COUNSELING INFORMATIONPatients with bronchial asthma, a history of bronchial asthma, severe chronic obstructive pulmonary disease, sinus bradycardia, second or third degree atrioventricular block, or cardiac failure ...
-
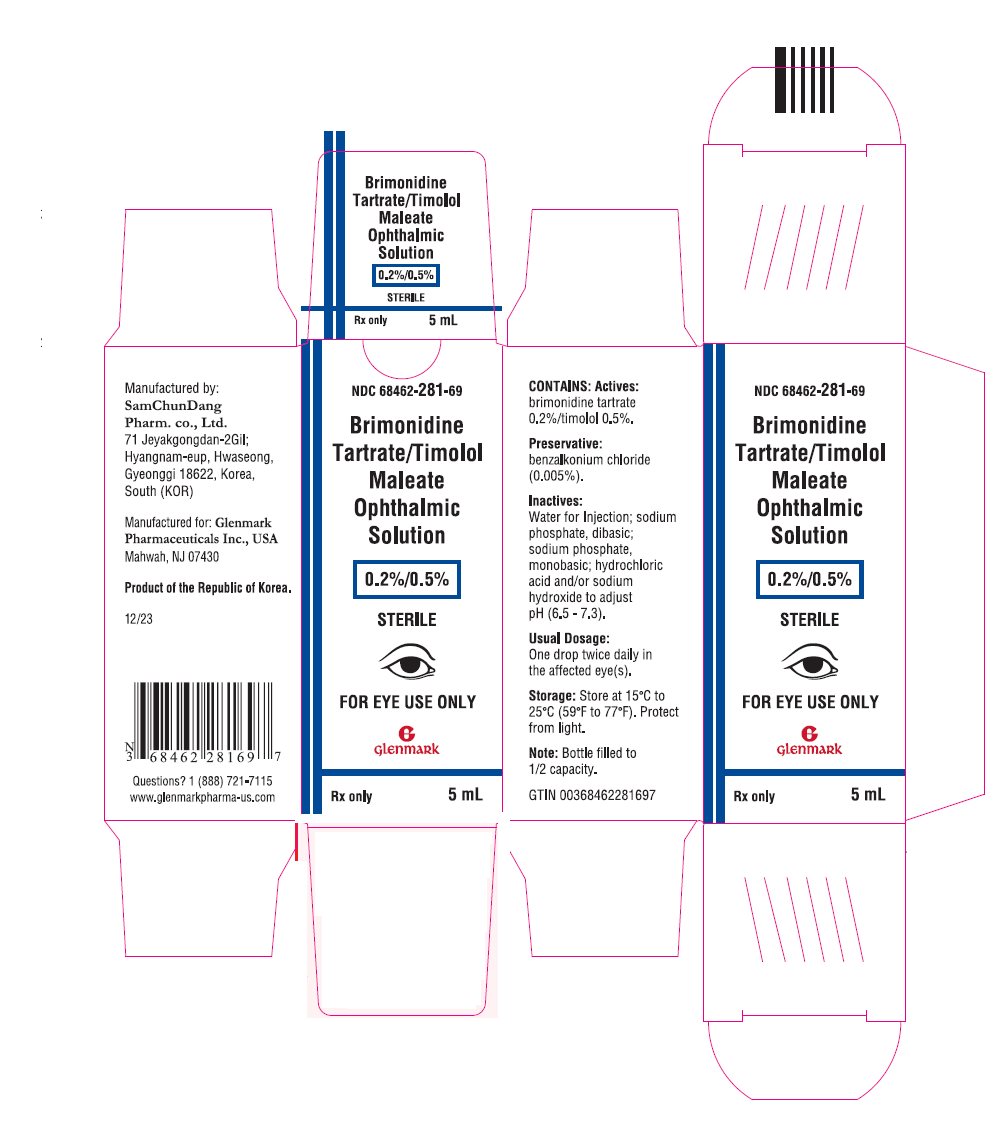
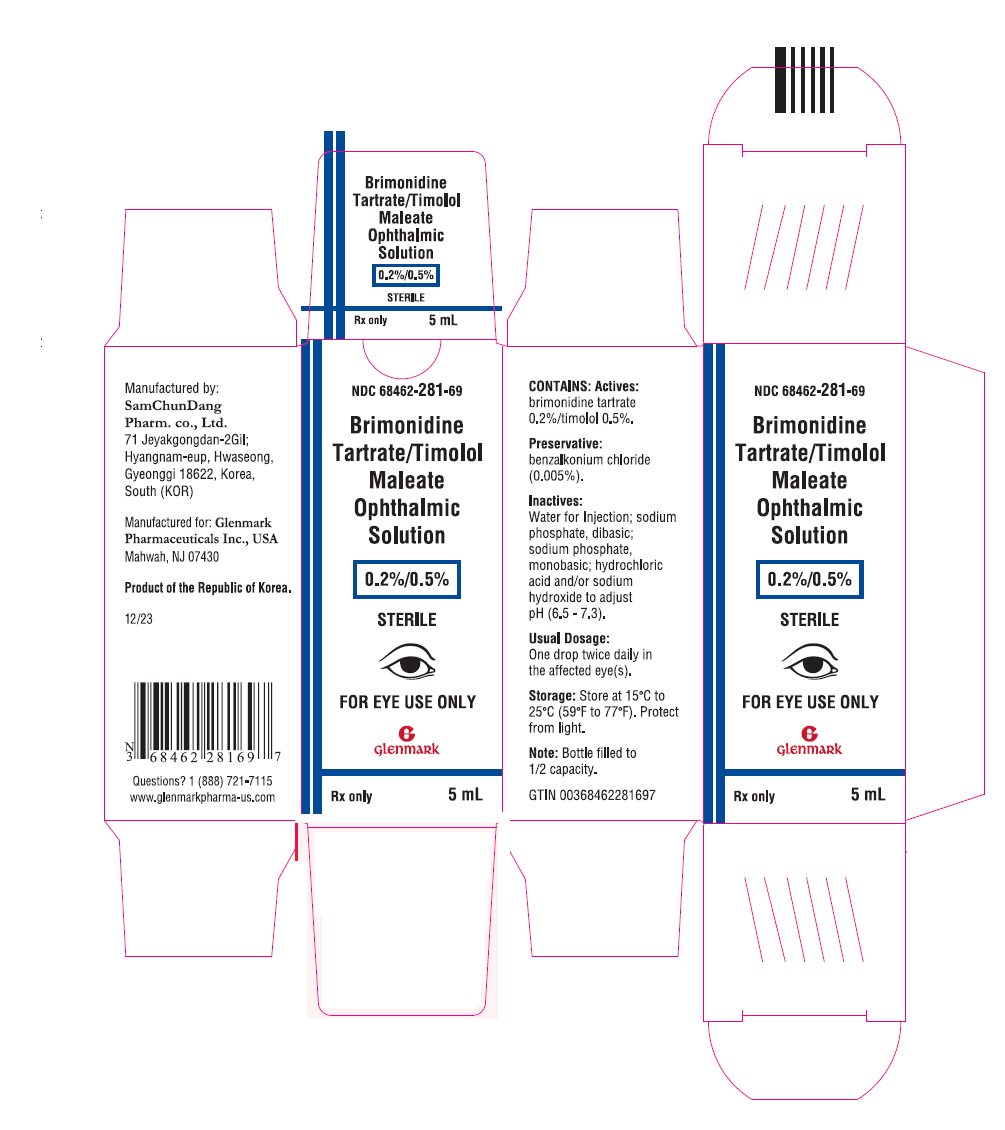
PRINCIPAL DISPLAY PANELNDC 68462-281-69 - Brimonidine Tartrate/Timolol Maleate Ophthalmic Solution - 0.2%/0.5% STERILE - FOR EYE USE ONLY - Rx only - 5 mL
-
Package/Label Display Panel NDC 68462-281-69 - Brimonidine Tartrate/Timolol Maleate Ophthalmic Solution - 0.2%/0.5% STERILE - FOR EYE USE ONLY - Rx only - 5 mL
-
INGREDIENTS AND APPEARANCEProduct Information